B21, China Town Mall, Midrand
Medical Orthopedic Mini Multifunctional Bone Drill Saw Oscillating Saw Adapter Power Electric Drill with adapters
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1600294843039-1711524936

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 03 Feb, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
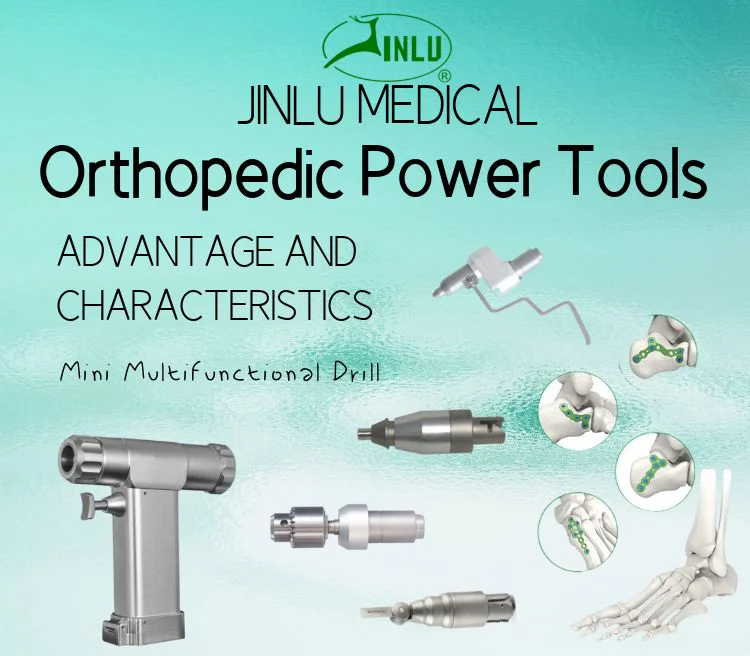
1. What is the Medical Orthopedic Mini Multifunctional Bone Drill Saw?
The JINLU SAW is a compact orthopedic surgical instrument combining a bone drill and oscillating saw functions with interchangeable adapters. It is designed for precision, adjustability and sterilization compatibility during orthopedic procedures.
2. What are the key specifications of this instrument?
Key specs include Ni‑MH battery (2100mAh), adjustable speed 0–1200 r.p.m., sterilization compatibility up to 135°C, weight ~5 kg, packaged in an aluminum box, and CE/ISO certification. Instrument classification: Class II.
3. What functions does the device provide?
It functions as a bone drill and an oscillating saw with multifunctional adapters to support a variety of orthopedic cutting and drilling tasks as required in surgical procedures.
4. What battery does it use and how long does the battery last?
The unit uses a Ni‑MH battery with a capacity of 2100mAh, which is specified to offer long‑lasting performance. Actual runtime depends on load and procedure; contact the supplier for typical runtimes under specific operating conditions.
5. What is the adjustable speed range and why is it important?
Speed is adjustable from 0 to 1200 r.p.m., allowing surgeons to tailor cutting/drilling speed for precision and tissue preservation during different procedural tasks.
6. Is the instrument sterilizable and what methods are supported?
The instrument (or sterilizable components) is rated for sterilization at temperatures up to 135°C. Follow the manufacturer's instructions for recommended sterilization methods (e.g., steam autoclave) and for guidance on which parts are autoclave‑safe.
7. Can the entire unit, including the battery, be autoclaved?
Manufacturers often require removal of batteries or electronic modules prior to autoclaving. Confirm with the product manual or supplier which components must be detached before sterilization to avoid damage.
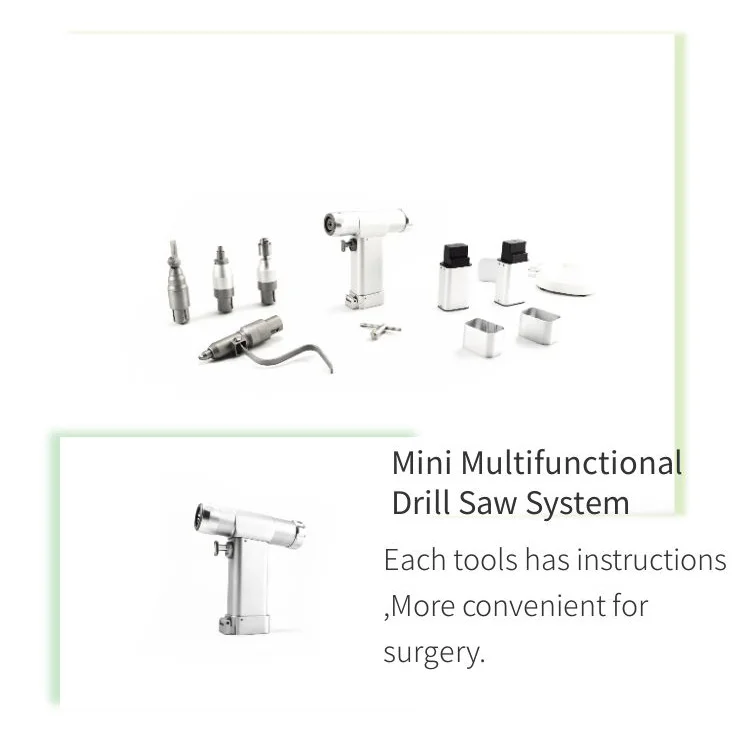
8. What accessories and adapters are included?
The product listing indicates it comes with adapters for drill and saw functionality, but exact accessory sets may vary. Request a detailed packing list from the seller to confirm included blades, bits, wrenches and spare parts.
9. Is this device CE/ISO certified and what classification does it have?
Yes. The instrument is CE and ISO certified and is classified as a Class II medical device.
10. How much does the device weigh and is it portable?
The instrument weighs approximately 5 kg, designed to be lightweight for easy handling and maneuverability in the operating room.
11. What is the minimum order quantity (MOQ) and shipping port?
The listed minimum order quantity is 1 set and the shipping port indicated is Shanghai, China.
12. Is the saw/drill compatible with other electric drill systems?
Compatibility with third‑party drill systems depends on adapter interfaces and specifications. Verify mechanical and electrical compatibility with the supplier before attempting to use with other systems.
13. Who should operate this instrument?
This instrument is intended for use by qualified and trained surgical personnel within an appropriate clinical setting. It should be used according to institutional protocols and the manufacturer's instructions.
14. What maintenance or cleaning procedures are recommended?
Follow the manufacturer's maintenance instructions: clean gross contaminants before sterilization, inspect blades/adapters for wear, replace damaged components, and remove batteries or electronic modules when required for sterilization or servicing. Schedule regular servicing as recommended.
15. What warranty and after‑sales support are available?
Warranty and after‑sales service terms are not specified in the basic listing. Contact the supplier or manufacturer for detailed warranty coverage, service centers, spare parts availability and technical support options.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals