B21, China Town Mall, Midrand
Portable accurate Digital Rechargeable Spo2 Handheld Pulse Oximeter Fingertip Pulse Oximeter
- Section : Medical Supplies
- Category : Pulse Oximeters
- SKU : 1600902163299

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 03 Feb, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
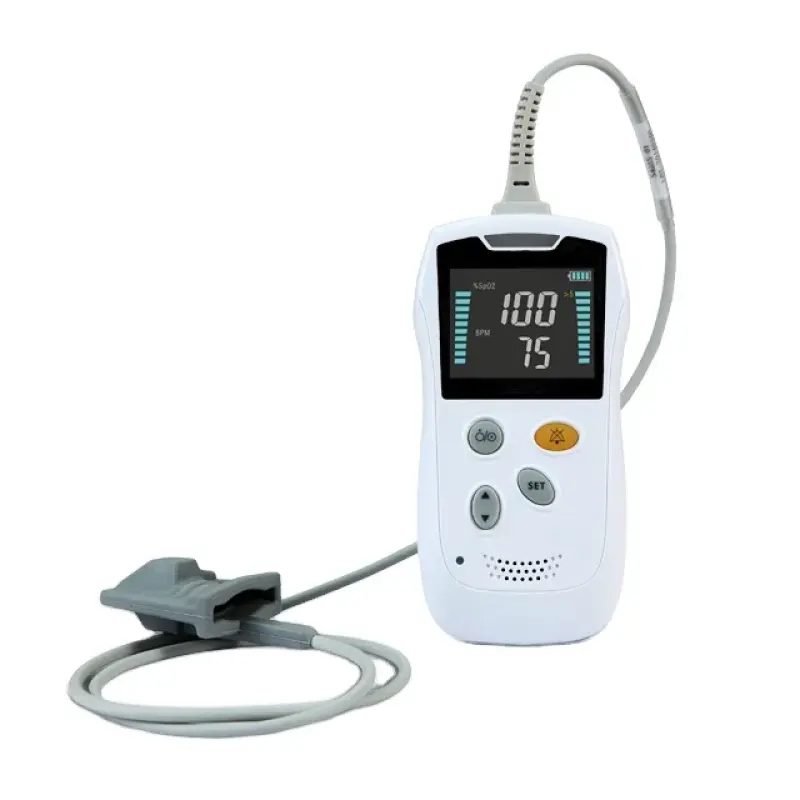
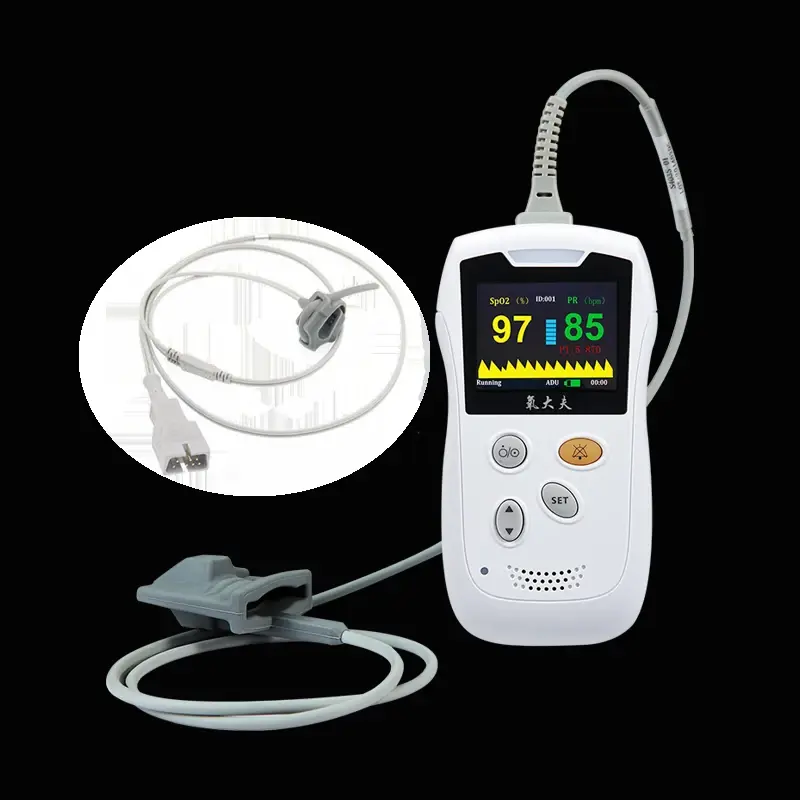
1. What measurements does the HS10A Portable Handheld Pulse Oximeter provide?
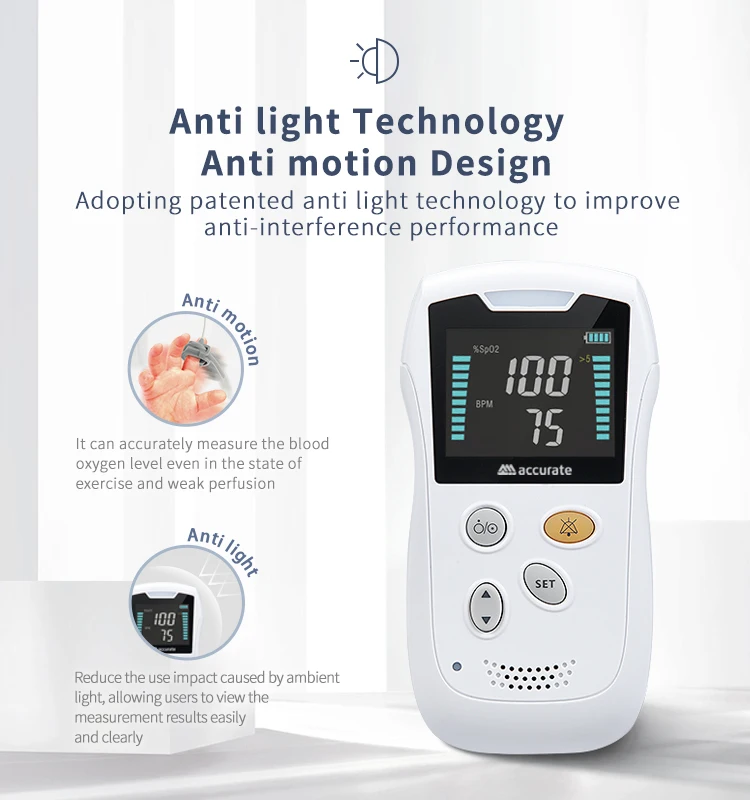
The HS10A measures blood oxygen saturation (SpO₂) and pulse rate, displaying both values clearly on its screen for spot checks or ongoing monitoring.
2. Is the HS10A medically certified?
Yes. The HS10A is CE-certified and carries ISO and CFDA certifications. It is classified as a Class II medical device and manufactured under EN ISO13485:2016 quality standards.
3. How accurate are the readings?
The HS10A is designed for reliable and precise monitoring suitable for clinical and home use. For clinical interpretation or treatment decisions, always correlate readings with clinical signs and consult a healthcare professional.
4. Can the HS10A be used for both adults and children?
Yes. The unit is suitable for pediatric and adult monitoring. However, it may not fit very small neonates or preterm infants—please check fit and consult your clinician for neonatal use.
5. What powers the device and how do I replace the battery?
The HS10A uses a removable battery (power supply mode: removable battery). To replace it, open the battery compartment, remove the depleted battery, and insert a fresh approved battery according to polarity markings. Refer to the user manual for battery type and disposal instructions.
6. Does the device have alarms?
Yes. The HS10A includes built-in audible alerts that notify you when measurements exceed preset thresholds. See the user manual for information on alarm behavior and how to set or adjust threshold values (if applicable).
7. How do I clean and disinfect the pulse oximeter?
Wipe the exterior and sensor areas with a soft cloth dampened with a mild detergent or 70% isopropyl alcohol. Do not immerse the device in liquid or use abrasive cleaners. Allow the device to dry fully before use.
8. How should I store the HS10A when not in use?
Store the device in a cool, dry place away from direct sunlight and excessive heat or humidity. Avoid storing in dusty or corrosive environments. Remove the battery for long-term storage to prevent leakage.
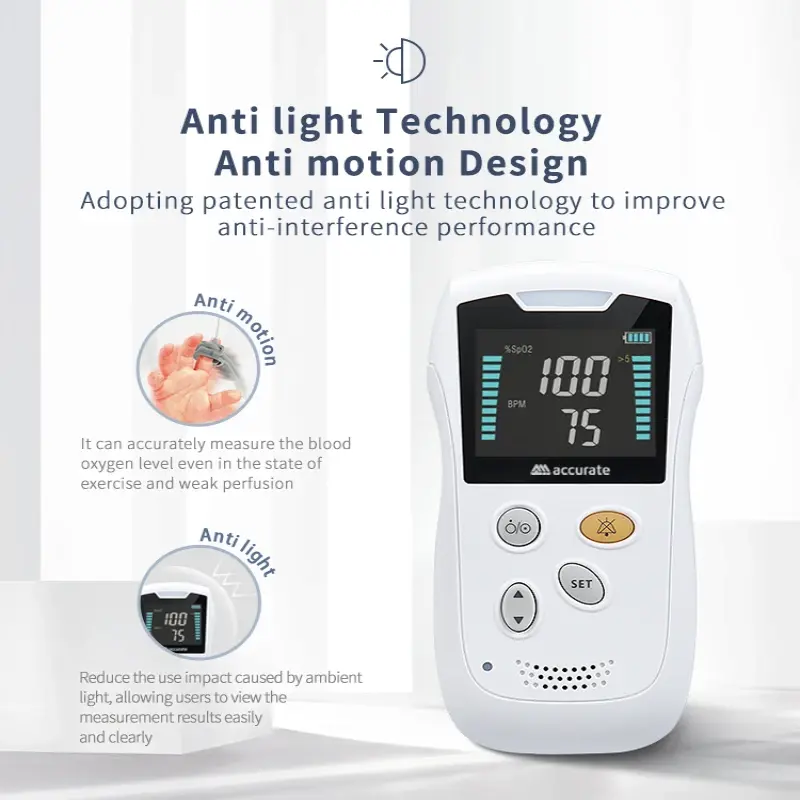
9. What can cause inaccurate readings?
Common causes include poor circulation or low perfusion, excessive motion, cold or dirty fingers, nail polish or artificial nails, ambient light interference, and incorrect finger placement. Reposition the finger, warm the extremity, or remove nail coverings and retake the measurement.
10. Does the HS10A require calibration?
The HS10A is factory-calibrated and intended to be used without routine user calibration. Healthcare facilities may follow institutional procedures for periodic performance checks or verification against reference devices.
11. What is the device warranty and shelf life?
The HS10A comes with a 1-year warranty. The listed shelf life is 1 year; please verify the manufacture or expiry date on the packaging and contact the supplier for support if beyond the stated shelf life.
12. Is the HS10A suitable for continuous monitoring in a clinical setting?
The HS10A is suitable for routine monitoring in hospitals, clinics, and home settings. For continuous intensive monitoring or advanced clinical applications, consult clinical guidelines and consider device specifications and institutional requirements.
13. Are there any contraindications or safety precautions?
Avoid using the device as the sole basis for critical clinical decisions. Do not use on patients with suspected compromised peripheral perfusion without clinical correlation. Keep away from strong electromagnetic fields and follow all user manual safety instructions.
14. What should I do if the device displays an error or will not power on?
First check the battery for correct installation and charge/condition; replace if necessary. Ensure the sensor area is clean and the finger is positioned correctly. If the issue persists, consult the troubleshooting section of the user manual or contact the supplier/service center for assistance.
15. How should I dispose of the HS10A and its batteries?
Dispose of the device and batteries according to local electronic waste and hazardous material regulations. Do not discard batteries in household trash—use designated battery recycling or disposal facilities.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals