B21, China Town Mall, Midrand
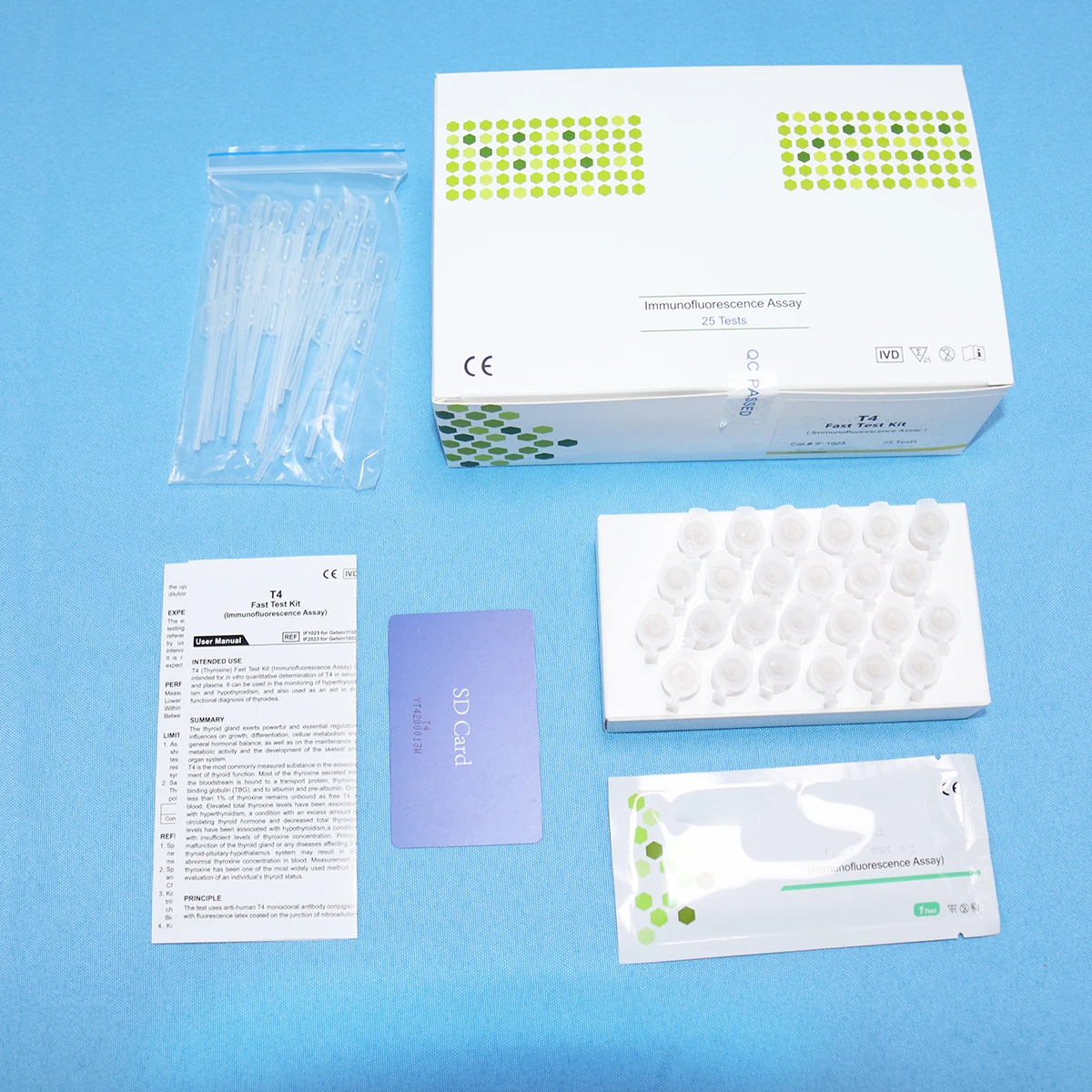
Medical Semi-Auto Blood Chemistry Analyzer – MSLIF08 Model
- Section : Medical Supplies
- Category : Clinical Analytical Instruments
- SKU : 1600897131482

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 26 Mar, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
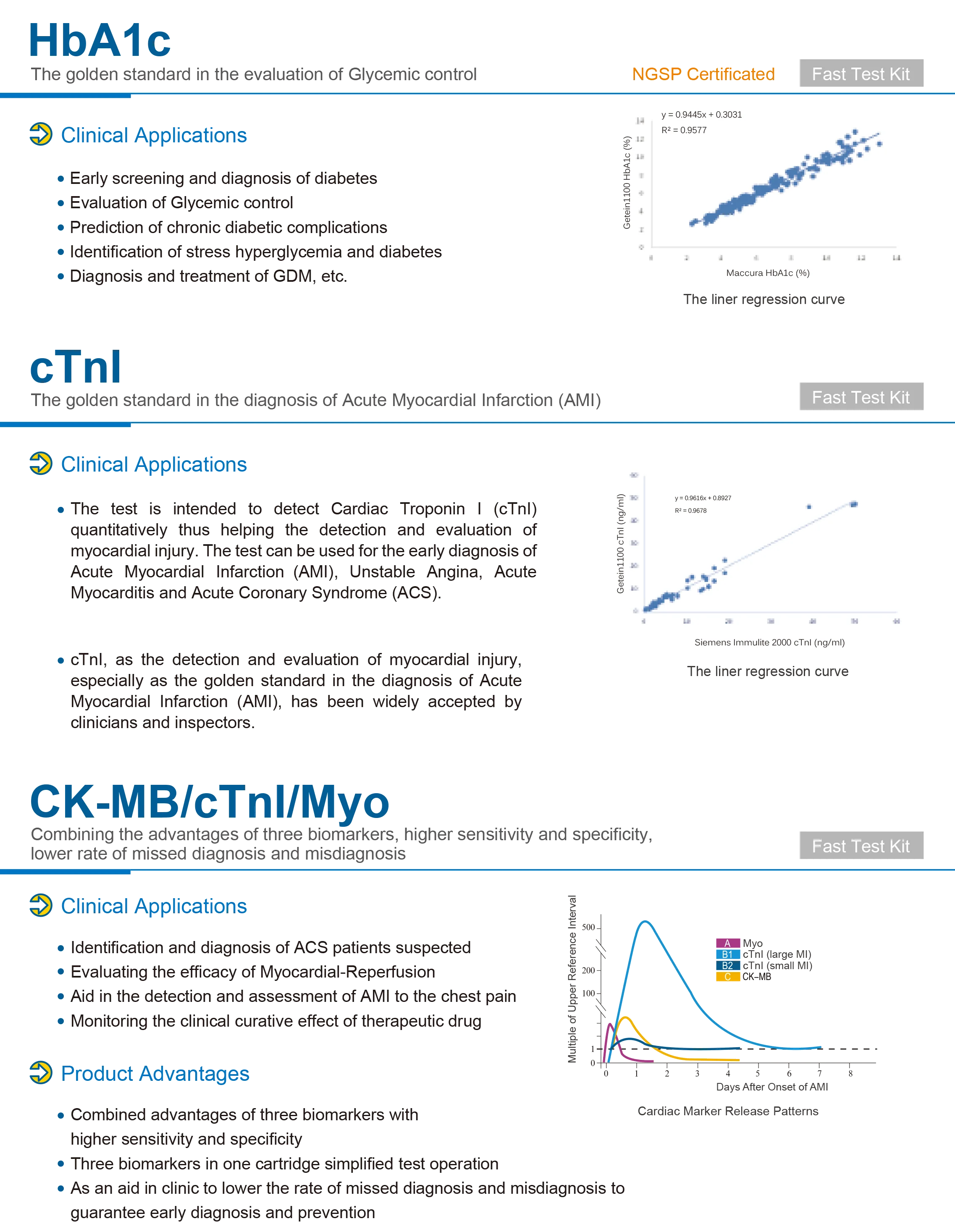
1. What is the MSLIF08 Medical Semi‑Auto Blood Chemistry Analyzer?
The MSLIF08 is a Class II medical semi‑automatic blood chemistry analyzer designed for clinical and laboratory biochemical testing. It provides high‑resolution photometric measurements, supports multiple wavelengths, and includes a 7‑inch touchscreen and internal thermal printer for routine diagnostic workflows.
2. What assay methods does the MSLIF08 support?
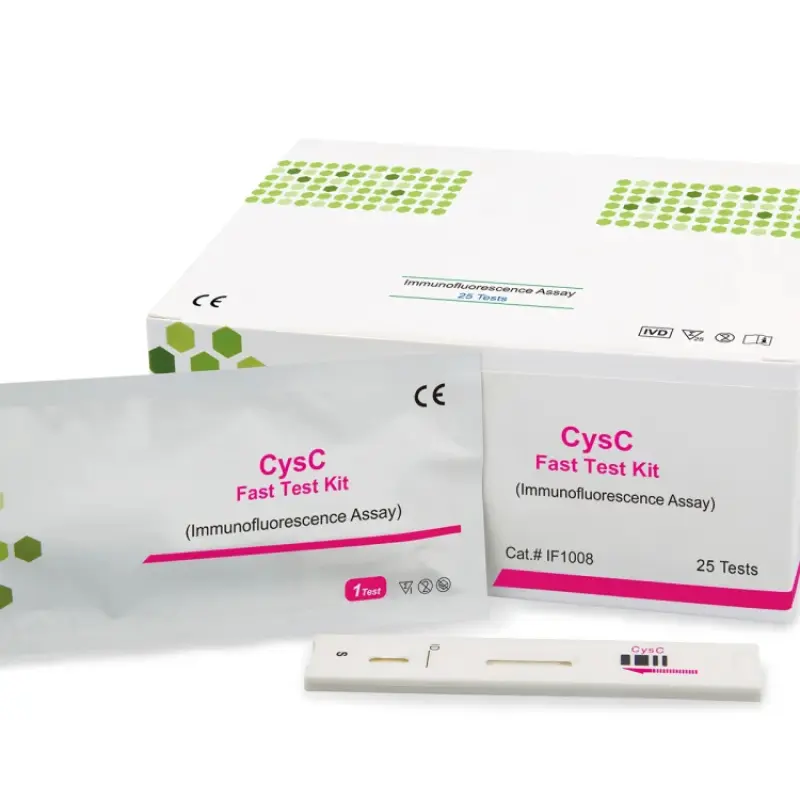
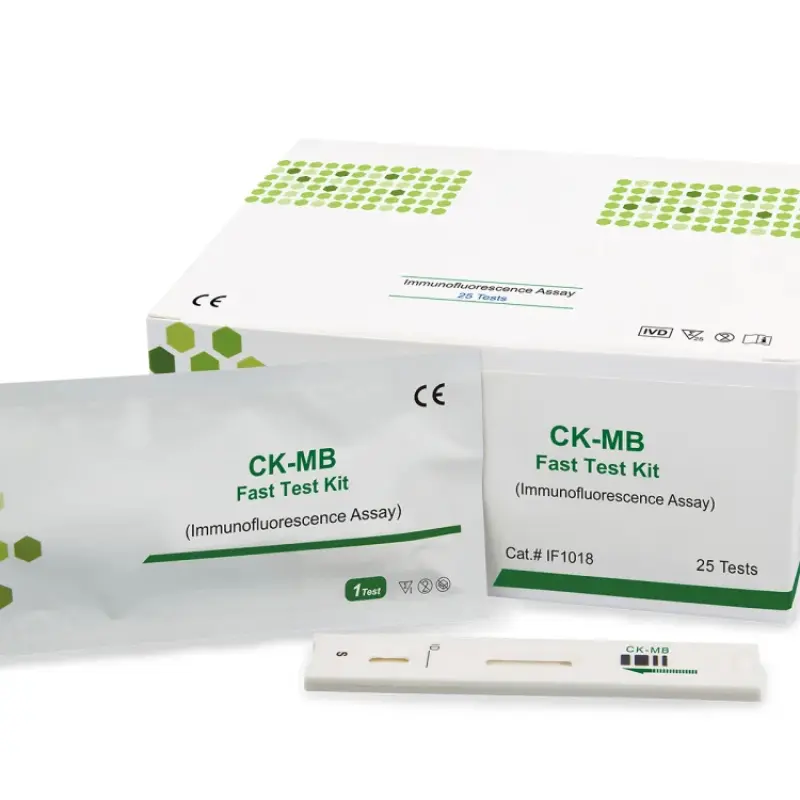
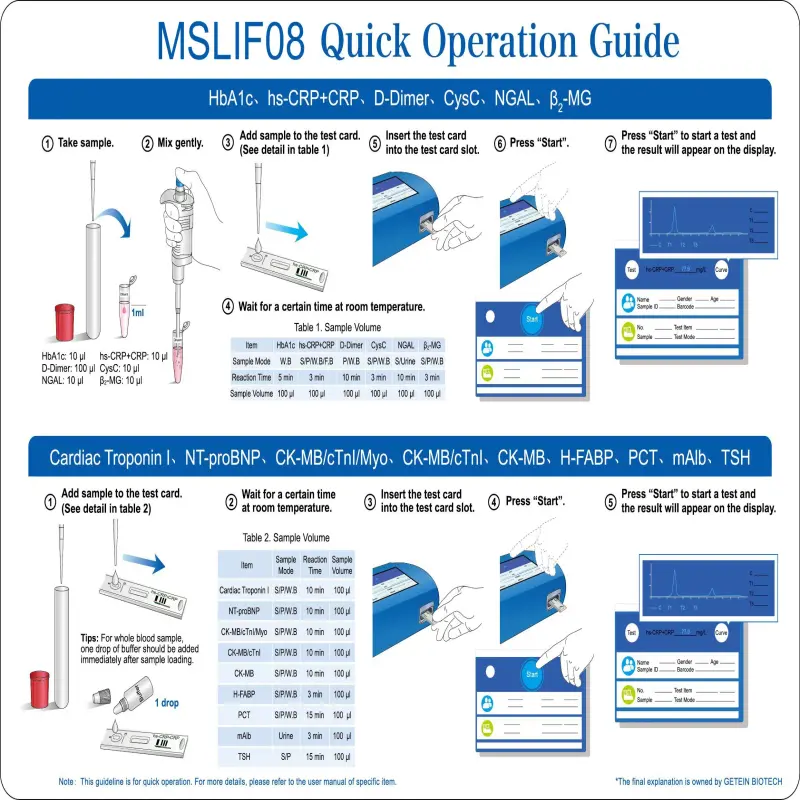
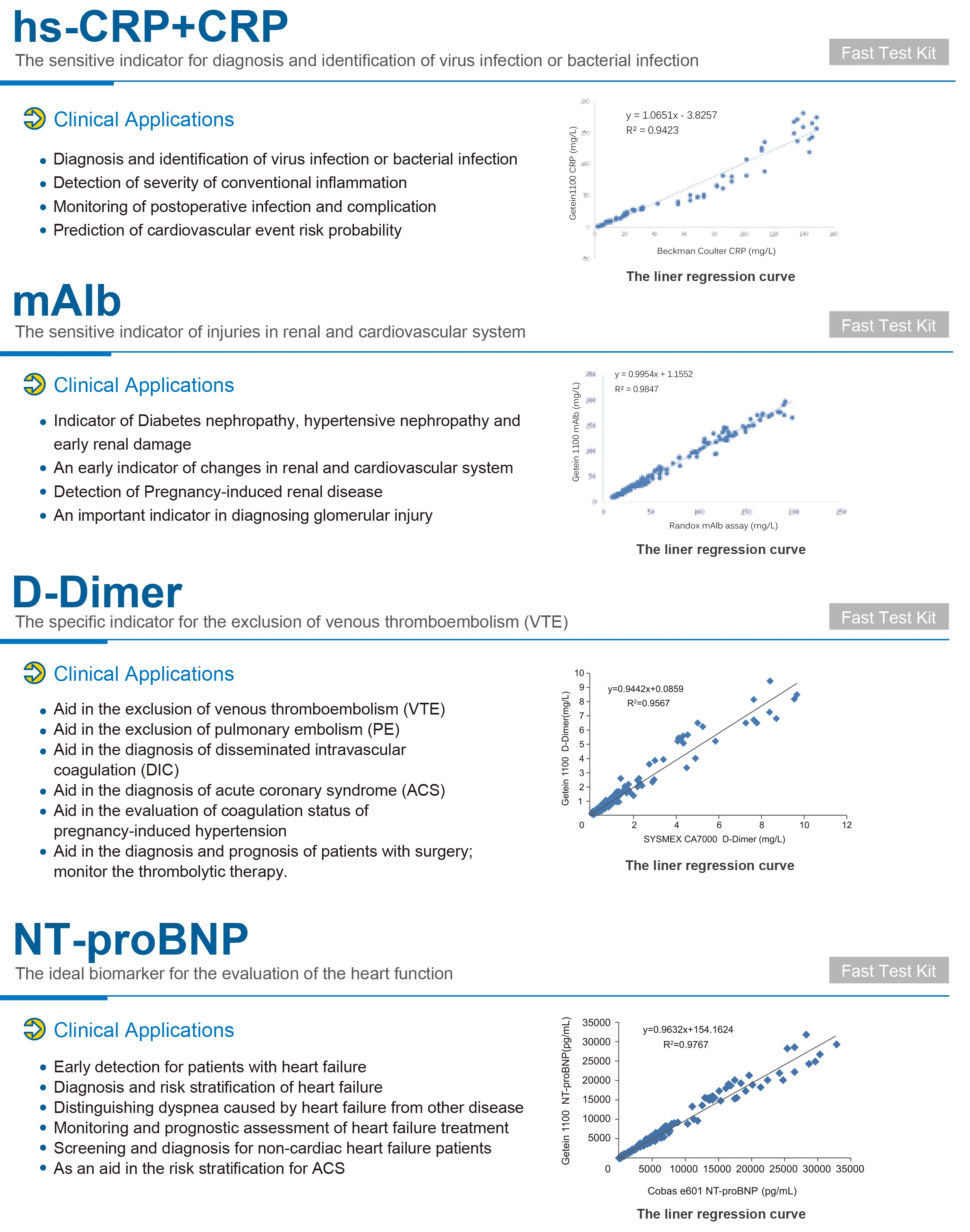
The product specification lists lateral flow chromatography (immunofluorescence) as an assay method. The unit also performs photometric biochemical measurements using a halogen light source and multiple wavelength filters. For compatibility with specific test kits or assay formats, confirm with the supplier or the user manual.
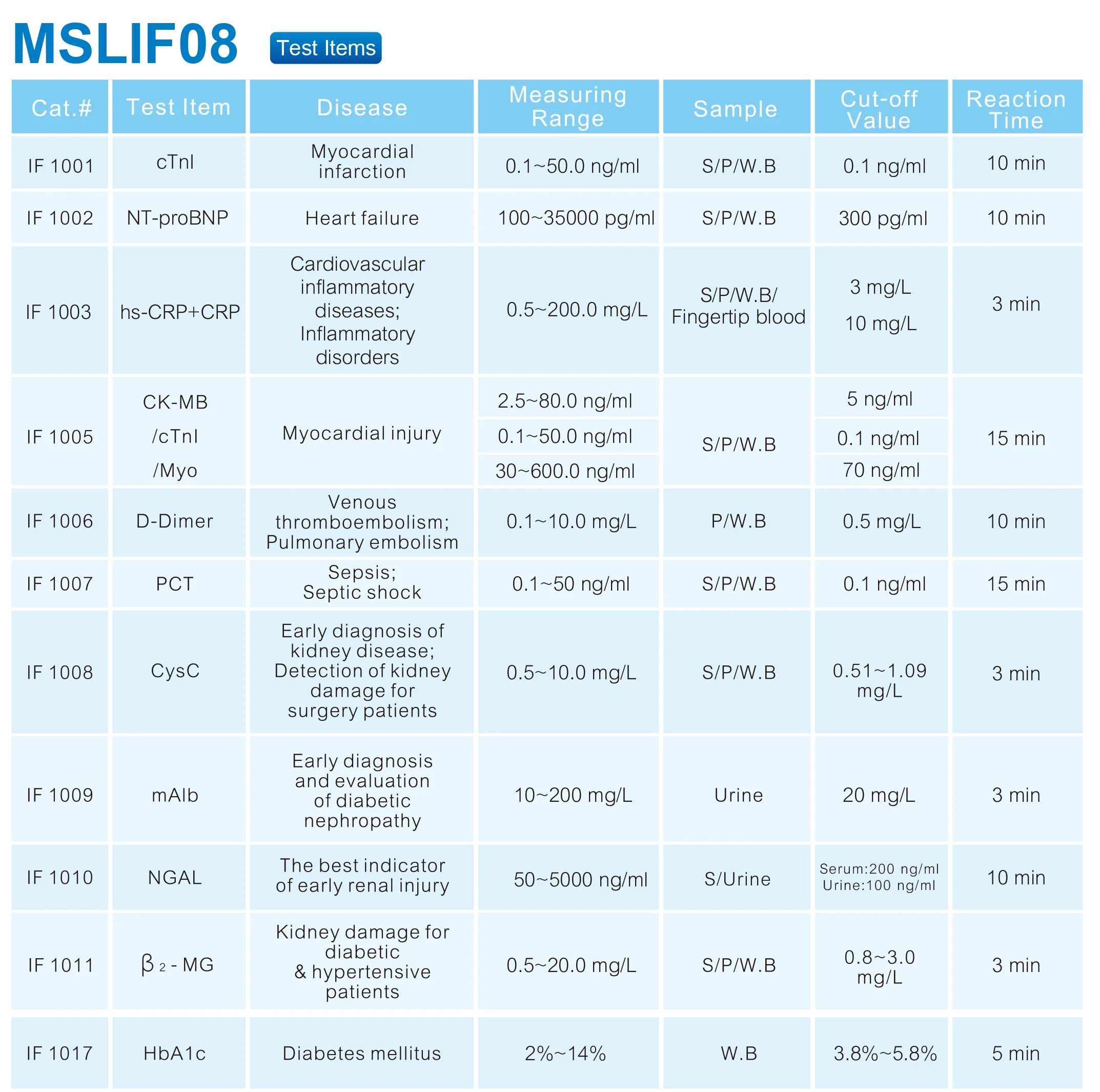
3. Which wavelengths are available and how accurate are they?
The analyzer supports wavelengths of 340, 405, 500, 546 and 620 nm, with three additional filter slots available for extra options. Wavelength accuracy is ±2 nm and the bandwidth is ≤10 nm, ensuring precise spectral specificity for typical biochemical assays.
4. What is the analytical resolution and sensitivity?
Displayed resolution is 0.001 Abs and calculated resolution is 0.0001 Abs, providing high‑resolution absorbance readings suitable for clinical chemistry tests.
5. What are the instrument dimensions and weight?
The product information contains two sets of physical data: one lists net weight 7 kg and dimensions 360 × 318 × 160 mm, while the specification page lists weight 1.9 kg and dimensions 261 × 241 × 115 mm. Please confirm the exact model/package dimensions and weight with your supplier prior to purchase or installation.
6. What are the power and environment requirements?
Power supply compatibility is AC100–240V, 50–60 Hz, 60 VA (specification). The working environment recommended temperature is +15 °C to +35 °C, humidity 10%–85%, and air pressure 70.0–106.0 kPa.
7. How many results can the analyzer store and what languages does it support?
The unit can store up to 10,000 records (maximum). The user interface supports English and Chinese.
8. What sample types can be used with the MSLIF08?
The analyzer is intended for routine blood chemistry testing, which commonly uses serum or plasma. Always follow the assay kit instructions and the user manual for recommended sample types and preparation.
9. How does the MSLIF08 minimize cross‑contamination between samples?
The analyzer specification states a cross‑contamination rate of ≤1%. To maintain low cross‑contamination, follow the recommended cleaning procedures, replace consumable reaction vessels or cuvettes as instructed, and adhere to sample handling best practices.
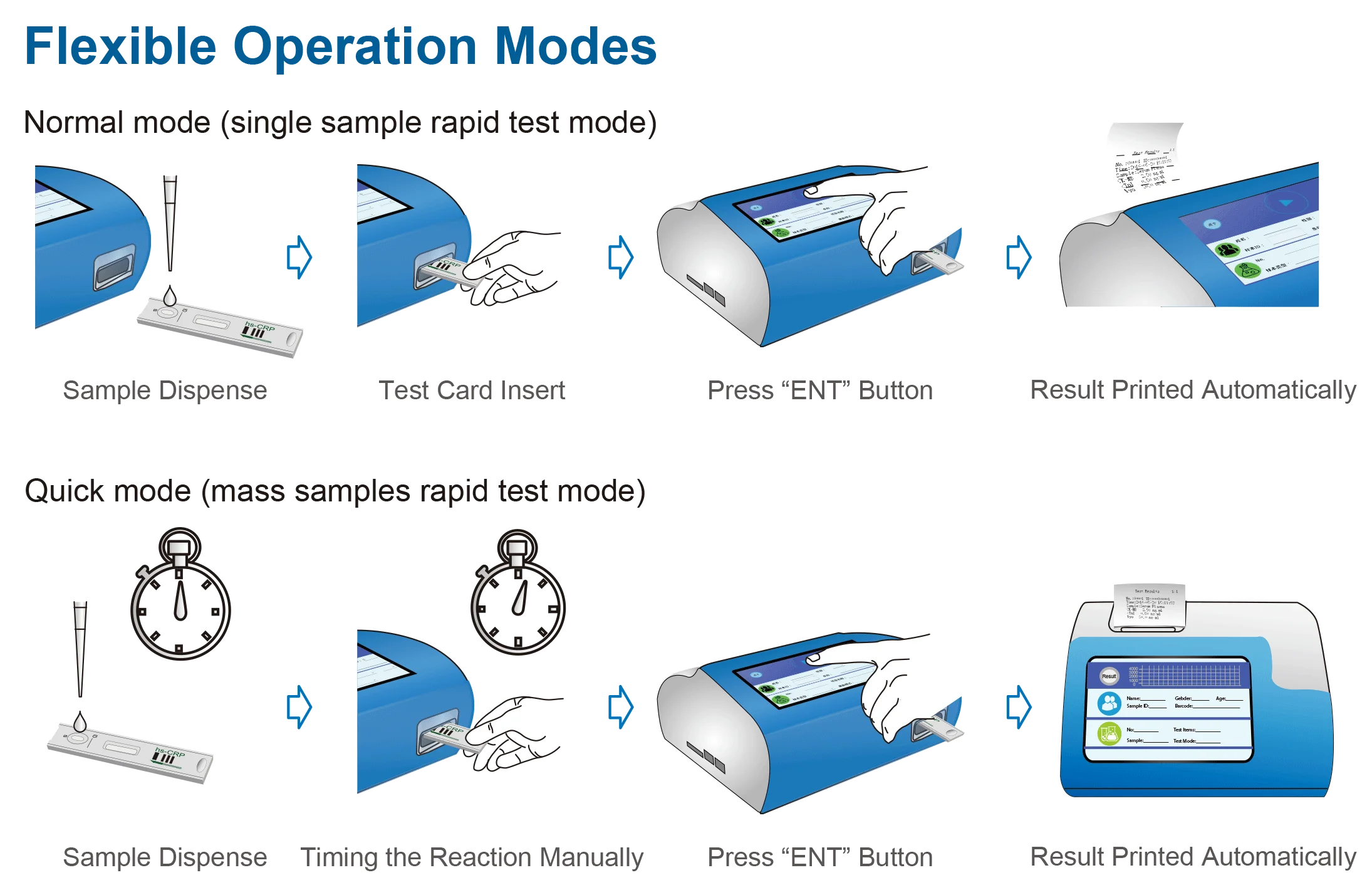
10. Does the MSLIF08 include a printer and how are results output?
Yes — the unit includes an internal thermal printer for immediate hard‑copy results. For electronic data export options (USB, network, etc.), consult the supplier or the technical manual, as electronic connectivity is not explicitly detailed in the provided specification.
11. Is the device suitable for point‑of‑care or small laboratory use?
Yes. The MSLIF08 is compact and described as lightweight and easy to install, making it suitable for small clinical labs, outpatient clinics, and other near‑patient testing environments. Confirm the exact footprint/weight with the supplier if space is constrained.
12. What maintenance and calibration are required?
Routine maintenance typically includes cleaning optical components and sample holders, replacing the halogen lamp when its intensity drops, periodic wavelength/photometric calibration using reference standards, and following any scheduled checks in the user manual. Contact the manufacturer or authorized service provider for recommended calibration intervals and procedures.
13. What consumables and accessories are required?
Common consumables include reaction cuvettes or test strips (depending on assay format), reagent kits, replacement optical filters (three additional filters supported), and thermal printer paper. Verify specific consumables and kit compatibility with your supplier or the product documentation.
14. Is the MSLIF08 certified and can it be used clinically?
The device is described as a Class II medical instrument, indicating it meets certain clinical safety and performance standards. Regulatory approvals and certification requirements vary by country—confirm local registration, certifications, and reimbursement status with the manufacturer or distributor before clinical use.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading