B21, China Town Mall, Midrand
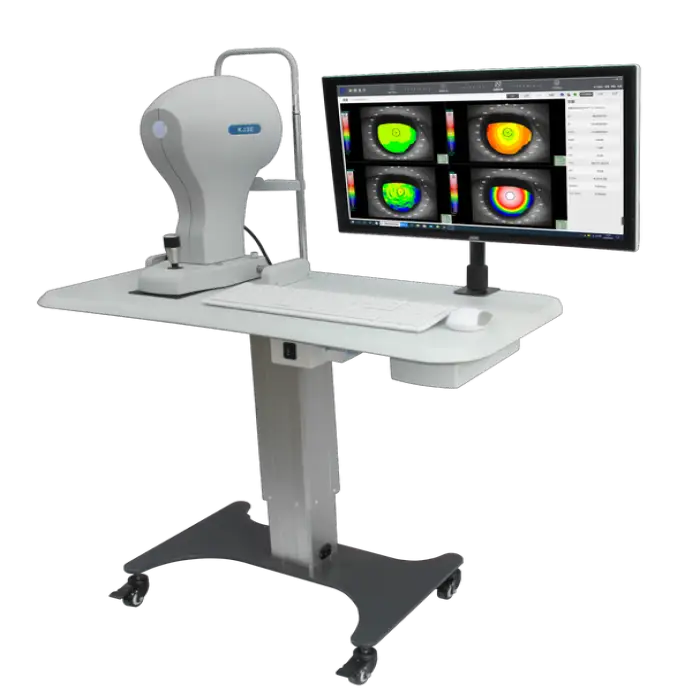
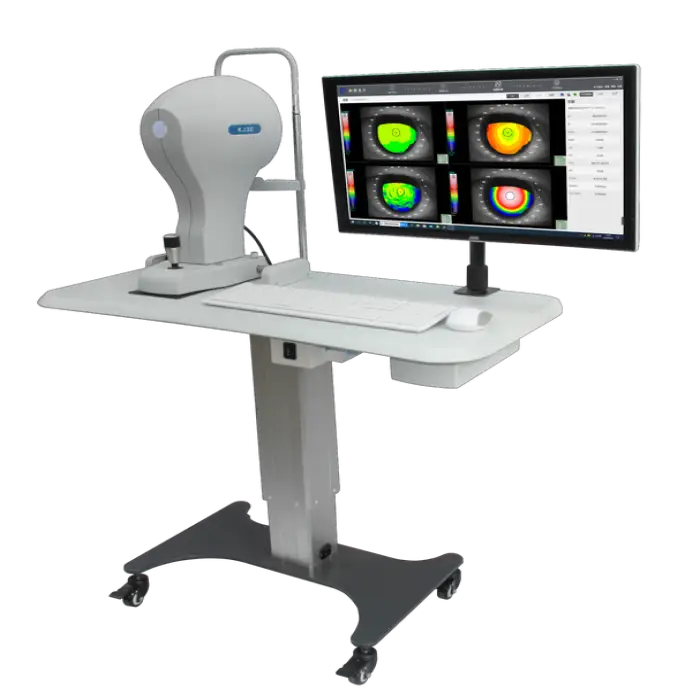
High-End Premium Corneal Topography Ophthalmic Equipment Electric Plastic
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1601432562130

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 06 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the High-End Premium Corneal Topography Ophthalmic Equipment?
It is a top-of-the-line corneal topography device (model KJ30 by Buywells) designed for precise mapping of the corneal surface and other optical examinations in clinical settings.
2. What are the primary clinical applications of this device?
The unit is used for corneal topography assessments, optical examinations, diagnosing corneal and refractive conditions, pre-operative evaluations for eye surgery, and monitoring patients' visual health over time.
3. What is the instrument classification and what does that mean?
The instrument is classified as Class I, indicating it is considered low risk under the manufacturer/supplier classification and intended for routine ophthalmic diagnostic use. Always follow local regulations and clinical protocols when using the device.
4. What are the key specifications I should know?
Key specs in the product listing: Brand Buywells, Model KJ30, electric power source, materials stainless steel and plastic, weight approximately 11 kg, shelf life 18 months, sold as a single item for ophthalmic optical examination.
5. How is the device powered?
The device is electrically powered. For mains voltage, plug type and voltage options please confirm with the supplier or the provided online technical support to ensure compatibility with your local power supply.
6. How portable is the equipment?
With a compact design and a weight of about 11 kg, the unit is relatively lightweight and convenient for clinical handling and repositioning within an exam room. It is not intended as a handheld unit.
7. What materials is the device made from and how durable is it?
The housing and structural parts use stainless steel and high-quality plastic. These materials provide good durability for normal clinical use; follow manufacturer care guidelines to maintain longevity.
8. What does the listed 'shelf life' of 18 months mean?
The 18-month shelf life refers to the manufacturer's recommended period for storage prior to put-into-service or the period during which the device is guaranteed to meet storage-related specifications. For in-service lifetime, maintenance and usage conditions determine longevity—contact support for details.
9. What maintenance and calibration are required?
Regular maintenance and periodic calibration are recommended to ensure measurement accuracy. Specific intervals and procedures are provided by the manufacturer—use the included documentation or contact online technical support for calibration schedules and authorized service options.
10. How should I clean and disinfect the device?
Use approved, non-abrasive cleaners and disinfectants compatible with stainless steel and medical-grade plastics. Avoid harsh solvents that can damage surfaces or optics. Refer to the manufacturer's cleaning instructions or contact technical support for recommended agents and procedures.
11. Does the device include software, data export, or connectivity features?
The product listing does not specify software or connectivity details. Please consult the supplier or online technical support to confirm available software, data export formats, EMR integration, network connectivity, and compatible file types.
12. What is included in the package and are accessories supplied?
The listing states a single item selling unit but does not list specific accessories. Typical packages may include power cables, user manual, and basic setup items. Confirm with the seller for a full packing list and availability of optional accessories.
13. Is training provided for clinicians and technicians?
Online technical support is available for users. Training offerings vary by supplier—ask the vendor about online or on-site training, user manuals, and training materials when placing your order.
14. What warranty and service options are available?
Warranty and service terms are not detailed in the product description. Please contact the seller or Buywells directly to obtain warranty duration, service contracts, spare parts availability, and authorized repair centers.
15. Are there any safety considerations or contraindications?
As a diagnostic ophthalmic instrument, it should be used by trained personnel following standard clinical safety procedures. There are no specific contraindications listed; however, always follow manufacturer instructions, patient comfort guidelines, and local clinical protocols when examining patients.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading