B21, China Town Mall, Midrand
Foot Scanner, Orthotic Insoles, Scanner Metal 3d Medical-Grade Foot Scan Machine - Diabetic Care & Posture Assessment Tool
- Section : Medical Supplies
- Category : Medical Consumables
- SKU : 1601377220829

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 09 Feb, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Foot Scanner and what does it do?
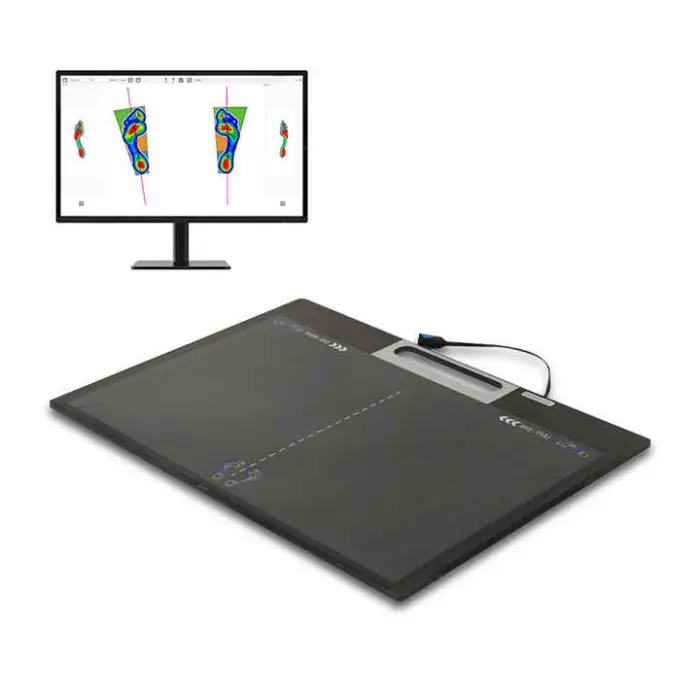
The Foot Scanner (Carotec) is a medical-grade, floor-mounted 3D foot scan machine that performs plantar pressure analysis to support diabetic foot care, posture assessment, and custom orthotic insole fitting. It provides automatic, easy-to-operate scans for clinical settings.
2. What clinical applications is this device intended for?
Typical applications include diabetic foot assessments and monitoring, posture and gait analysis, custom orthotic insole fitting, routine foot health evaluations in hospitals and clinics, and research into plantar pressure and foot biomechanics.
3. How does the 3D plantar pressure analysis work?
The device captures a 3D representation of the plantar surface and measures pressure distribution across the foot. The resulting pressure maps and 3D models help clinicians identify high-pressure areas, asymmetries, and postural issues to guide treatment or orthotic design.
4. How long does a scan take?
Scans use an Automatic Sensing (Auto Sense) mode for rapid acquisition. Typical scan time is very short (usually a matter of seconds), but actual duration depends on workflow and the software settings—ask the supplier for measured timings.
5. What are the physical dimensions and weight of the unit?
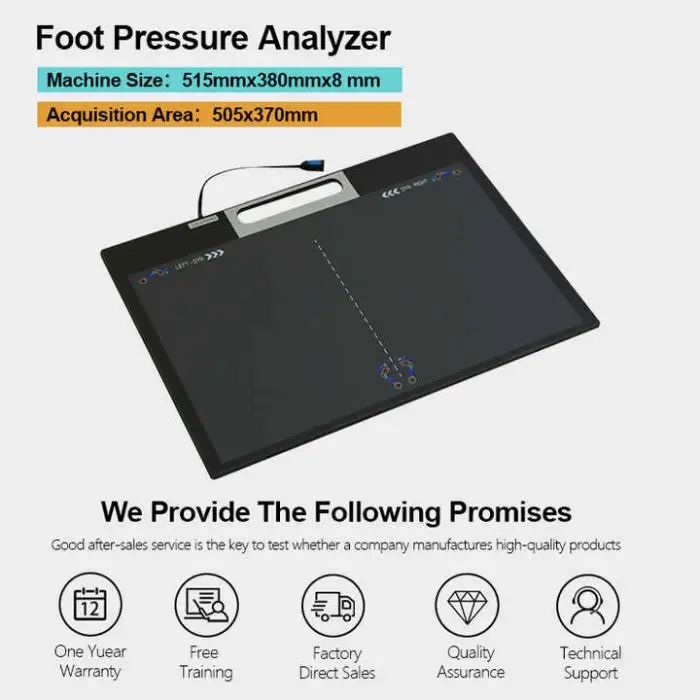
Single package size is 60 x 40 x 10 cm and the single gross weight is 5.000 kg (as listed).
6. How does the scanner connect to other systems?
The scanner provides a USB interface for data transfer. For details on supported file types, drivers, or SDK/API availability for integration, contact Carotec or your distributor.
7. What output formats are available (3D models, pressure data)?
The product description indicates 3D scans and plantar pressure analysis outputs. Exact export formats (e.g., STL, OBJ, CSV, TIFF) depend on the bundled software—confirm supported formats with the vendor.
8. Is this device suitable for diabetic patients?
Yes — the scanner is designed for diabetic foot care assessments and provides plantar pressure information useful for identifying ulceration risk. Use should follow established clinical protocols and the patient's care team guidance.
9. Is the scanner easy to use and does it require training?
The unit is advertised as having easy operation and Auto Sense scanning to minimize user steps. Some basic training is recommended for clinical staff to interpret pressure maps, integrate into workflows, and maintain the device properly; vendor training/support is typically available.
10. Where is the scanner mounted and what is its recommended environment?
This is a floor-mounted unit intended for hospital and clinic use. Install it on a stable, level surface in a clean clinical environment and follow the user manual for clearance and environmental requirements.
11. What are the power requirements and does it run from USB power?
The device provides a USB interface for data connectivity, but specific power requirements are not listed in the description. Many clinical scanners require mains power in addition to USB data connections—confirm exact power specs in the user manual or with Carotec.
12. What is the maximum patient weight the scanner supports?
A maximum supported patient weight is not specified in the provided specs. The device is designed for clinical use with adults, but please verify the rated weight limit with the manufacturer before use with heavier patients.
13. How should the scanner be cleaned and maintained?
General guidance: follow the manufacturer's cleaning and maintenance instructions. Typically this includes wiping surfaces with compatible disinfectants, avoiding liquid ingress into electronics, disconnecting power before maintenance, and performing periodic calibration/diagnostics as recommended by Carotec.
14. Is the device certified as medical-grade and does it have regulatory approvals?
The product is described as medical-grade in the listing. For specific regulatory certifications or approvals (e.g., CE, FDA clearance or local medical device registration), request certification documents from Carotec or your supplier to confirm compliance with your region's regulations.
15. What warranty, technical support, and software updates are available?
Warranty length, technical support, and software update policies are not specified in the description. Contact Carotec or your distributor for details on warranty coverage, available training, technical support channels, and software maintenance or upgrade plans.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals