B21, China Town Mall, Midrand
Electrosurgical Unit - ES-300D by Taktvoll
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1600313040186

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 26 Mar, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Electrosurgical Unit ES-300D by Taktvoll?
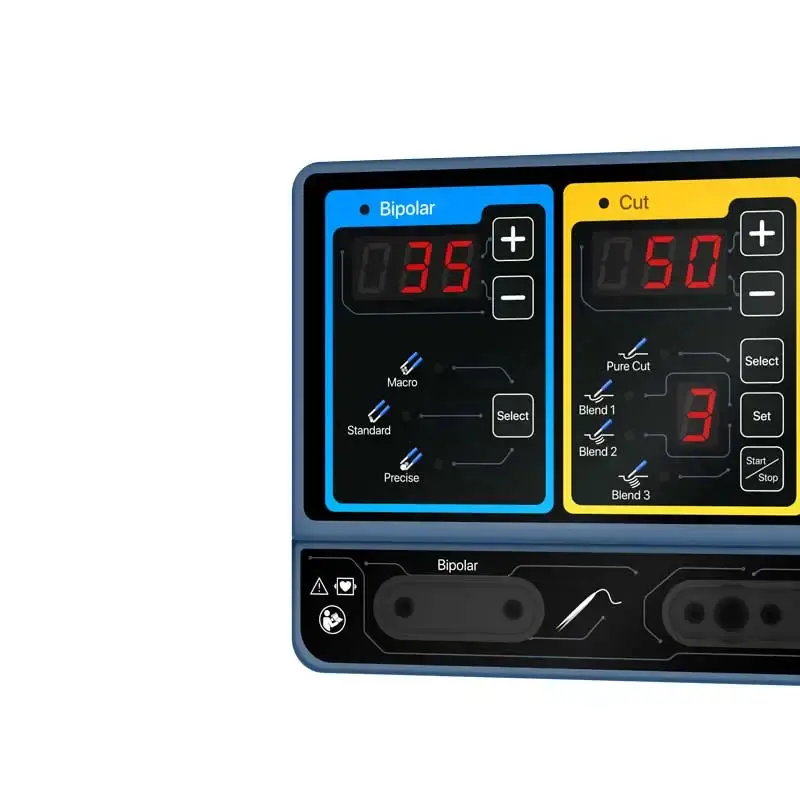
The ES-300D (also referenced as model ES-300DP) is an electrosurgical generator designed for precision cutting, coagulation and tissue dissection. It supports both monopolar and bipolar modes and is intended for use in a variety of surgical procedures.
2. Which monopolar and bipolar modes does the ES-300D provide?
Monopolar: two monopolar output ports with four cutting modes (pure cut, blend 1, blend 2, blend 3) and three coagulation modes (spray, forced, soft). Bipolar: three bipolar modes (macro, standard, fine) for controlled bipolar coagulation.
3. What is the CQM contact quality monitoring system?
CQM (Contact Quality Monitoring) continuously checks the quality of the return electrode/tissue contact and alerts the operator to poor contact or faults to reduce the risk of burns and improve procedural safety.
4. Can the ES-300D cut and coagulate at the same time?
Yes. The unit supports simultaneous cutting and coagulation, enabling combined tissue effects when clinically required.
5. What safety certifications and standards does this unit meet?
The ES-300D is CE certified and conforms to GB15979-2002 safety standards for medical devices. It is classified as an Instrument Class III device.
6. What are the power/source and shelf life details?
Power source: electric mains. Specific voltage and plug options depend on the configuration—contact your supplier for country-specific compatibility. Shelf life is specified as 1 year.
7. How does the memory and startup behavior work?
The ES-300D starts with the most recently used mode, power and parameters. It also provides nine user memory sets for quick recall of preferred power settings and modes.
8. What controls and accessories are used with the ES-300D?
Control options include electrosurgical pens and a foot switch. Use Taktvoll-approved electrodes, pens, return electrodes and footswitches for compatibility and safety.
9. Is the unit suitable for which types of surgical procedures?
Common applications include general surgery, vascular surgery, tissue coagulation and cutting, and surgical dissection. Use should be determined by the clinical team based on procedure requirements.
10. What maintenance and cleaning procedures are required?
Perform routine visual inspections and functional checks per the manufacturer's manual. Clean external surfaces according to instructions, inspect cables and connectors regularly, and have servicing or repairs performed only by authorized technicians.
11. Are there audible or visual alarms and can their volume be adjusted?
Yes. The ES-300D includes alarm/alert functions related to CQM and device faults. A volume adjustment function is provided to set audible alert levels per operating-room needs.
12. Can accessories be sterilized or are they single-use?
Accessory reprocessing depends on the specific item. Some accessories are single-use while others may be reusable and sterilizable. Always follow the accessory manufacturer's labeling and the ES-300D user manual for sterilization and reuse instructions.
13. Who is authorized to operate the ES-300D?
Only trained and qualified medical personnel who are familiar with electrosurgical techniques and the unit's operating manual should operate the device. Follow institutional policies and training requirements.
14. What warranty and after-sales support are available?
Warranty terms and after-sales support vary by distributor and region. Contact Taktvoll or your local authorized distributor for specific warranty length, service contracts and repair options.
15. How do I obtain consumables and technical support for the ES-300D?
Order consumables (electrodes, pens, footswitches) and request technical support through Taktvoll or an authorized local distributor. They can confirm part compatibility, shipment options and service arrangements.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading