B21, China Town Mall, Midrand
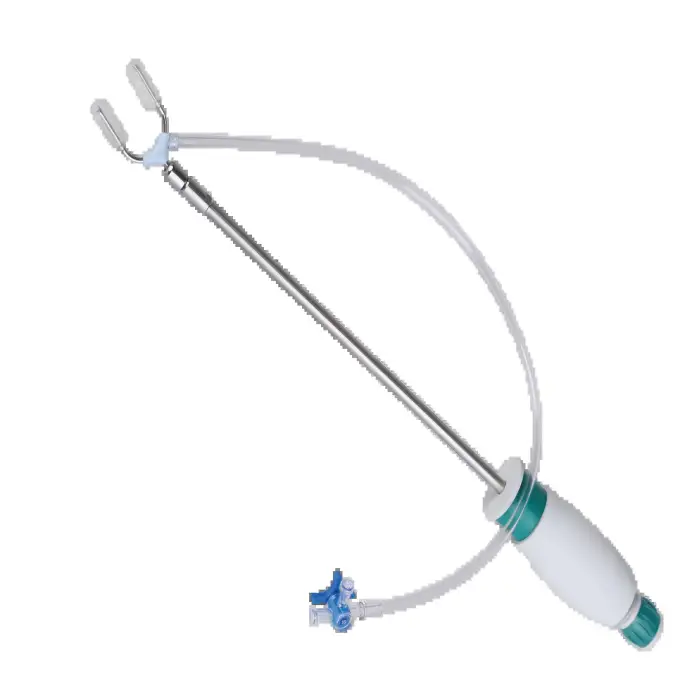
EEG - KT88 by CONTEC
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 225827463

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 26 Mar, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the EEG - KT88 by CONTEC?
The EEG - KT88 by CONTEC is a compact electroencephalogram (EEG) system designed for monitoring and recording brain electrical activity in hospitals, clinics, and other healthcare settings to assist in diagnosing neurological conditions.
2. What are the key features of the KT88 EEG system?
Key features include high-precision brain wave monitoring, a user-friendly interface for straightforward operation and quick analysis, a durable plastic housing suitable for clinical environments, and a portable form factor for use in multiple locations.
3. What clinical applications is the KT88 intended for?
The KT88 is intended for routine EEG monitoring and diagnostic support in conditions such as epilepsy, sleep disorders, and other cognitive or neurological impairments. It is intended for use by qualified clinical personnel.
4. What are the technical specifications I should know?
Manufacturer details: Brand — CONTEC; Model — KT88; Material — plastic; Instrument classification — Class IIP; Power source — electric (mains); Shelf life — 3 years; Quality certification — CE. For detailed electrical, channel, and performance specs, consult the product manual or vendor datasheet.
5. Is the KT88 portable and does it run on battery power?
The KT88 has a portable design that makes it easy to move between clinical locations. According to the product description, its power source is electric (mains). If a battery option is required, check with the supplier for available configurations or accessories.
6. What certification and regulatory status does the KT88 have?
The KT88 carries CE certification per the product information provided. It is listed as an Instrument Classification Class IIP medical device. For regulatory compliance in your country or facility, verify local registration and approvals with the manufacturer or distributor.
7. How do I set up and begin recording with the KT88?
Basic setup involves placing appropriate EEG electrodes on the patient per your facility’s protocol, connecting electrode leads to the device, plugging the unit into mains power, powering on, and following on-screen or manual instructions to begin acquisition. Always follow the supplied user manual and local clinical protocols.
8. What training is required to operate the KT88?
The KT88 is described as user-friendly, but operation should be performed by trained clinical personnel familiar with EEG acquisition, electrode placement, artifact recognition, and interpretation workflows. Manufacturers or distributors often offer training—contact CONTEC or your supplier for available courses or materials.
9. How should I clean and maintain the KT88?
Clean the external plastic housing and connectors with manufacturer-recommended disinfectants and procedures to avoid damage. Inspect electrode leads and connectors regularly for wear. Follow the maintenance schedule and instructions in the user manual; contact the manufacturer for service or repairs.
10. What accessories and consumables are required?
Typical accessories include EEG electrodes (disposable or reusable), electrode caps, lead wires, conductive gel or paste, and patient cables. Packaging contents may vary—confirm which accessories are included with your purchase and order consumables separately as needed.
11. How long is the shelf life and what does it mean?
The listed shelf life for the KT88 is 3 years. Shelf life refers to the period during which the unused device is expected to remain within manufacturer specifications when stored under recommended conditions. Check labeling and storage instructions to ensure viability.
12. How is patient data handled and exported?
Data handling and export capabilities may vary by software/firmware version. For specifics on data formats, storage, network connectivity, and export procedures, consult the product manual or contact CONTEC or your supplier. Always follow local data protection and patient privacy regulations when storing or transferring EEG data.
13. Are there any contraindications or safety warnings?
There are no device-specific contraindications listed in the brief product description. However, EEG should be used according to clinical guidelines. Follow all safety instructions in the user manual, ensure proper electrode application to avoid skin irritation, and observe standard electrical safety precautions for mains-powered medical equipment.
14. Where can I get warranty, service, and technical support?
Warranty terms, service plans, and technical support options are provided by CONTEC or the authorized distributor. Contact your supplier or CONTEC directly to obtain warranty details, request service, or access technical assistance and spare parts.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading