B21, China Town Mall, Midrand
CPM Knee Joint Trainer Stroke Injury Rehabilitation Therapy Device Leg Stretching Bending Training Treatment Machine
- Section : Medical Supplies
- Category : Household Medical Devices
- SKU : 1601367767411

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 05 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
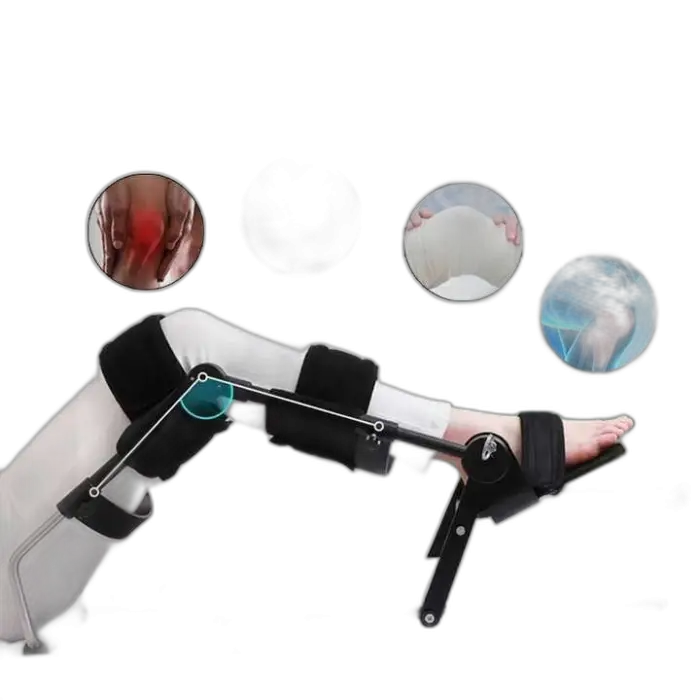
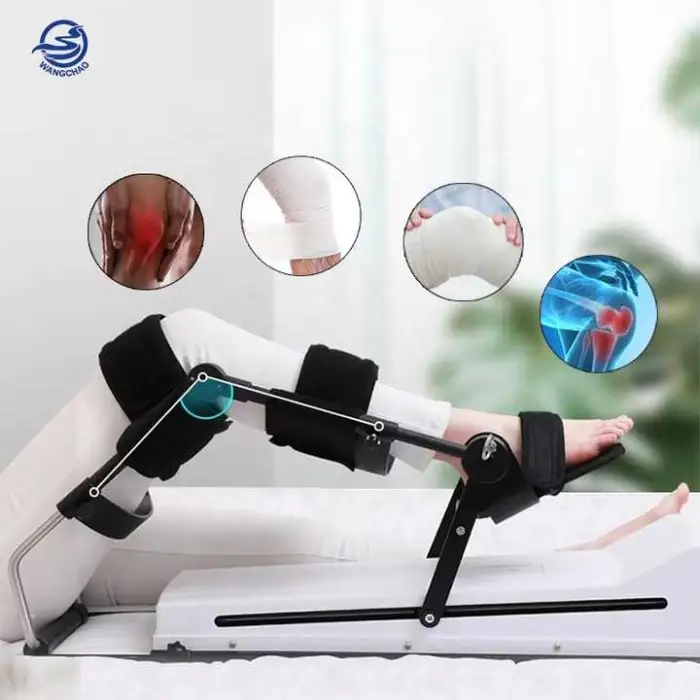
1. What is the English CPM Knee Joint Trainer?
It is a continuous passive motion (CPM) device designed to gently and repeatedly stretch and bend the knee to support rehabilitation after stroke, surgery, or knee injury. It provides passive movement to improve joint mobility and aid recovery.
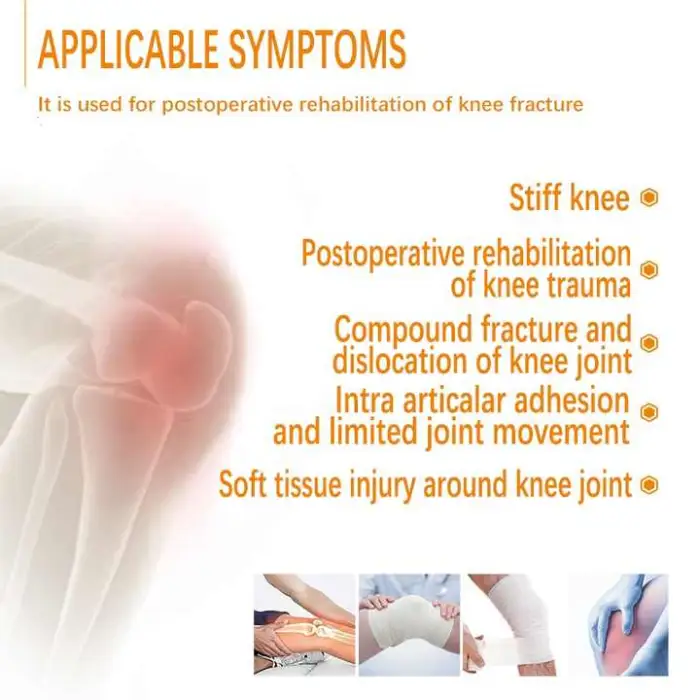
2. Who is this device intended for?
People recovering from knee surgery, stroke-related immobility, ligament or soft-tissue injury, or chronic stiffness may benefit. Use should be guided by a physician or physiotherapist to ensure it fits the patient’s clinical needs.
3. How does CPM therapy help knee recovery?
CPM promotes gradual joint motion to reduce stiffness, help prevent adhesions and contractures, maintain range of motion, and support circulation and tissue healing when used as part of a rehabilitation plan.
4. What sizes are available and how do I choose the right one?
The trainer comes in S, M and L sizes. To choose the correct size, compare your leg measurements (thigh and calf circumference and leg length) to the seller’s sizing chart or consult your therapist or the vendor for guidance.
5. Is it suitable for home use?
Yes. The device is designed for use at home, in clinics, rehab centers and hospitals. Home use should follow instructions from a healthcare professional and the product manual.
6. Is the device portable?
Yes. It has a lightweight, portable design. The single package dimensions are 124 x 42 x 38 cm and the gross weight is 14.800 kg, which makes it reasonably easy to move or transport.
7. What materials is the trainer made from?
The device is constructed from plastic and metal components for durability and hygiene.
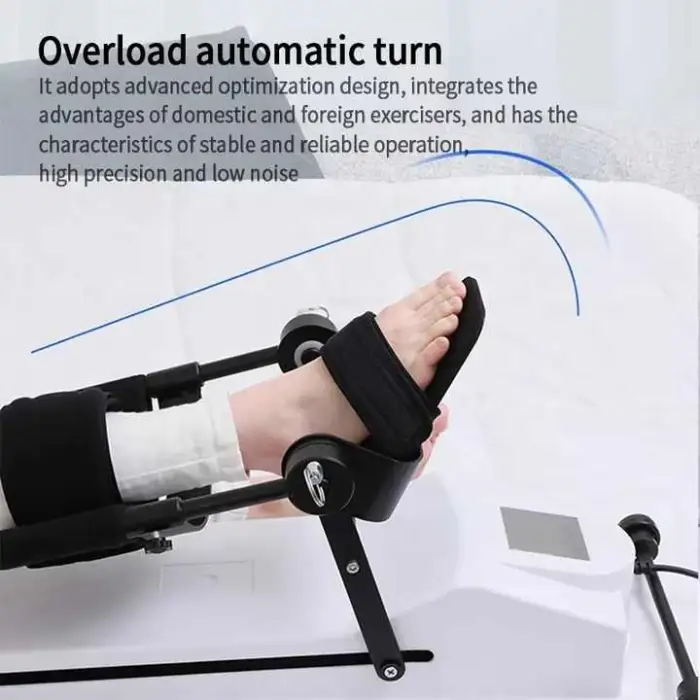
8. How is the unit powered and controlled?
This is a motorized CPM trainer. Specific power requirements, voltage and control functions (speed/angle adjustments) are provided in the product manual—check the seller’s specifications or included documentation for exact details.
9. How long and how often should I use the device?
Treatment duration and frequency vary by condition. Typical protocols used by clinicians range from short sessions (e.g., 15–60 minutes) multiple times per day. Always follow the treatment plan prescribed by your doctor or therapist.
10. Are there any contraindications or safety precautions?
Yes. Do not use if you have untreated deep vein thrombosis (DVT), active infection at the joint, unstable fractures, severe spasticity, or where motion is medically contraindicated. Stop use and consult a clinician if you experience increased pain, swelling, numbness or unusual symptoms.
11. Do I need a prescription to purchase or use it?
Requirements vary by region and healthcare policy. Many users buy CPM devices without a prescription, but clinical supervision for use is strongly recommended. Check local regulations and consult your healthcare provider.
12. How do I assemble and set up the trainer?
Minimal assembly is typically required. Follow the step-by-step instructions in the product manual. If you are unsure, ask a caregiver or contact the vendor for assembly support or demonstration.
13. How should I clean and maintain the device?
Wipe surfaces with a soft cloth and mild disinfectant; avoid soaking or spraying water into the motor/controls. Regularly inspect fasteners, straps and moving parts for wear and keep the unit stored in a dry, dust-free area. Refer to the maintenance section of the manual for detailed care instructions.
14. What warranty, spare parts or service support are available?
Warranty terms and after-sales service vary by seller. Check the product listing or contact the vendor or manufacturer for warranty duration, available spare parts and authorized service centers.
15. How should I transport and store the device when not in use?
For transport, use the original packaging if possible and lift with assistance if needed (the packed weight is about 14.8 kg). Store in a cool, dry place away from direct sunlight and extreme temperatures. Secure moving parts to prevent damage during transport.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading