B21, China Town Mall, Midrand
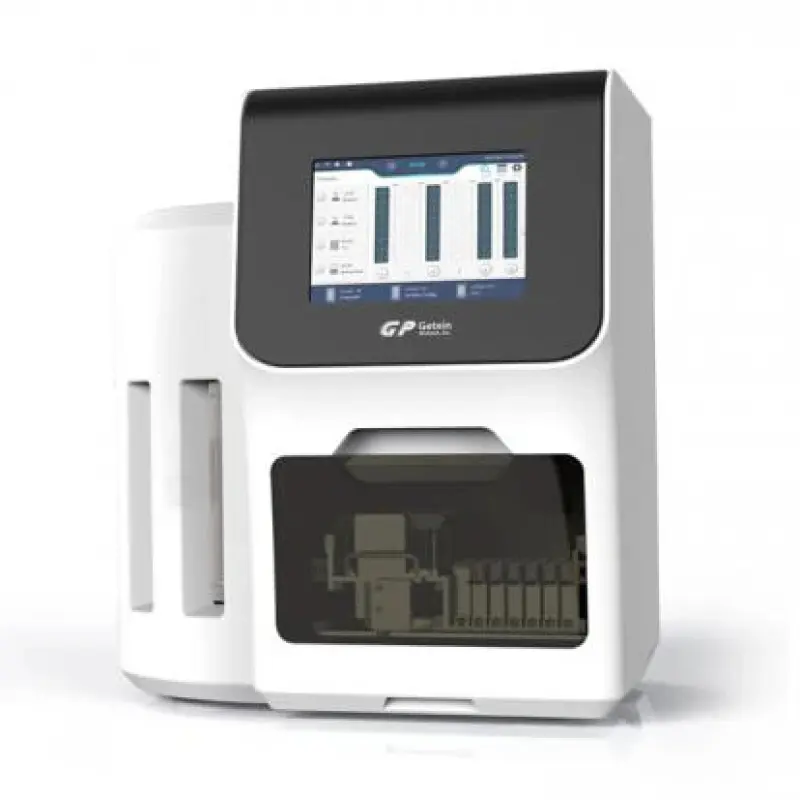
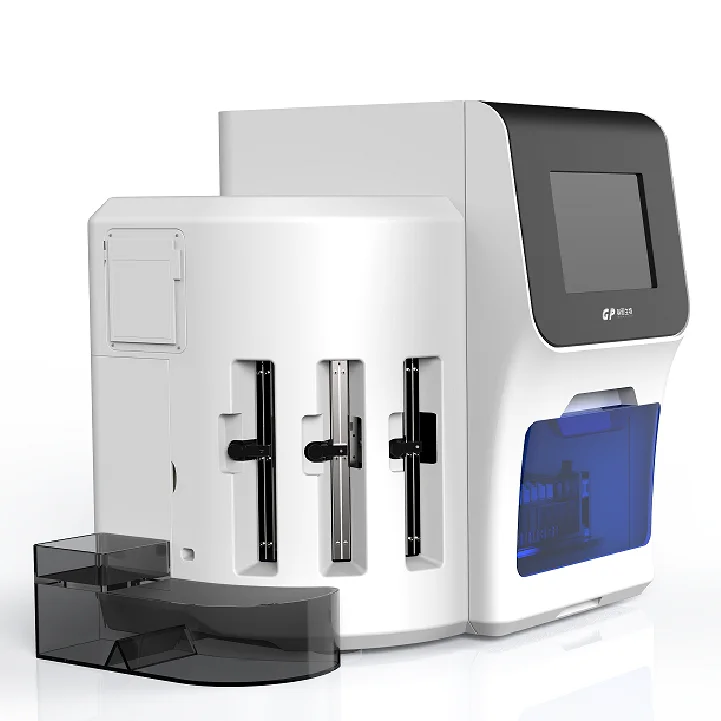
Blood analyzer machine Getein biotech 1600
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1600891618639

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 05 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
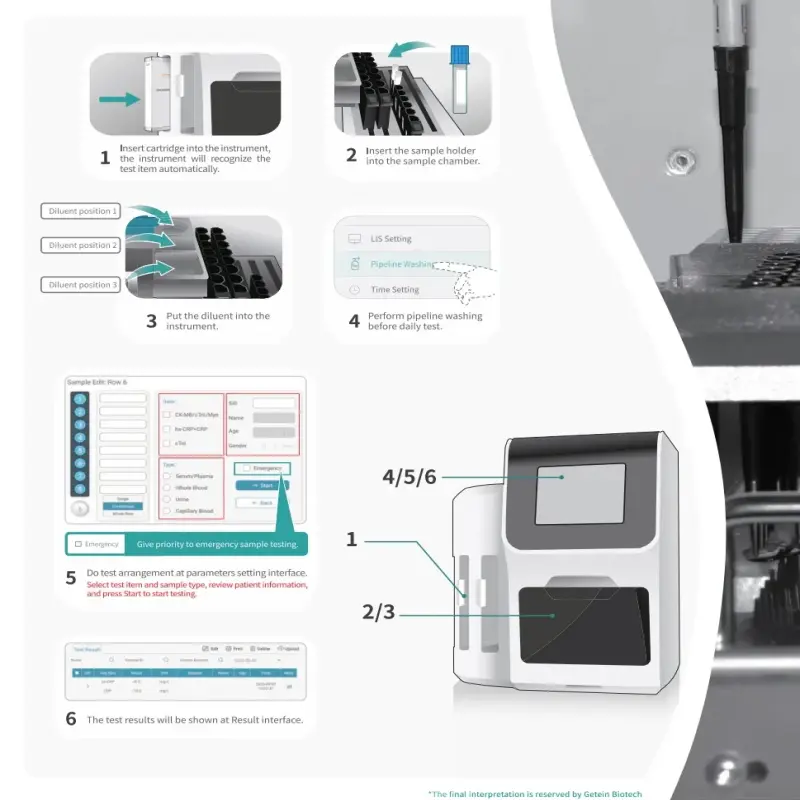
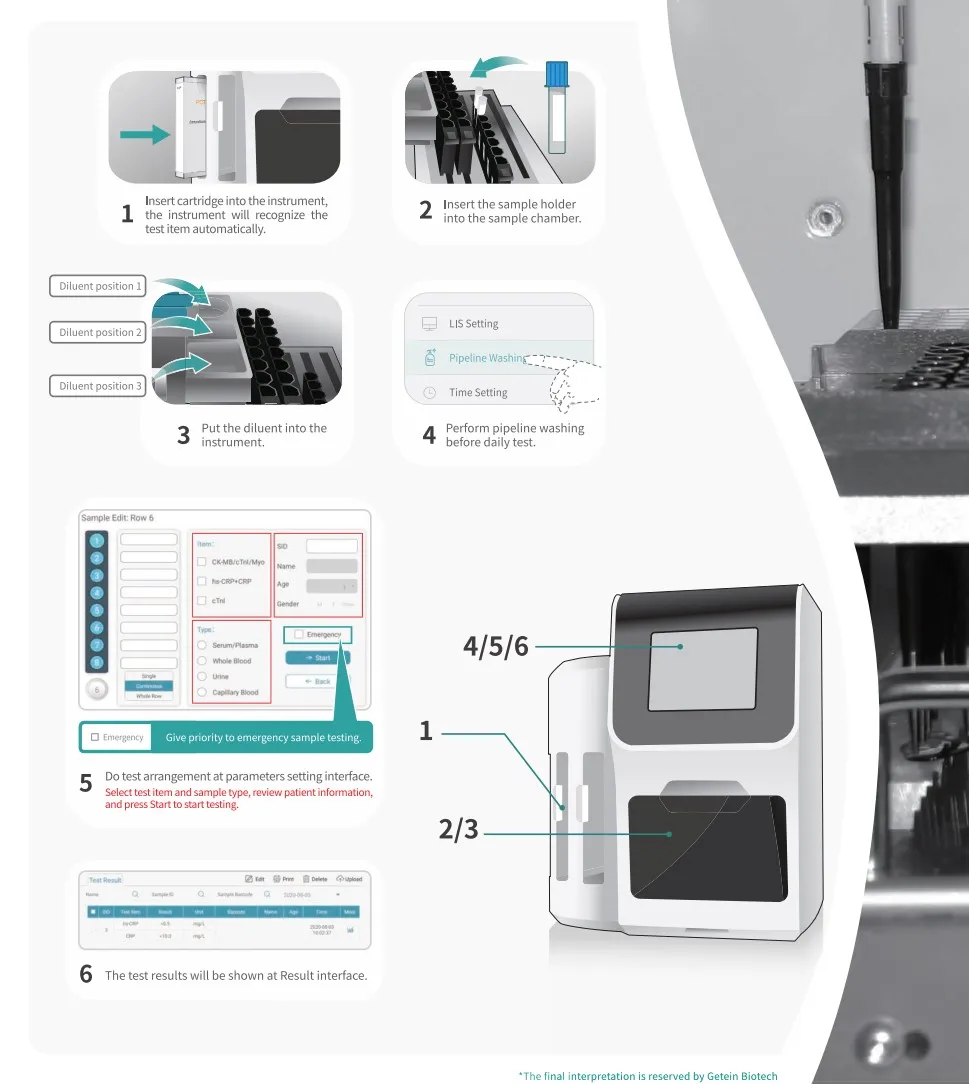
1. What is the Getein 1600 Immunofluorescence Quantitative Analyzer?
The Getein 1600 is a fully-automatic bench-top immunofluorescence quantitative analyzer that uses lateral flow chromatography to provide rapid, quantitative results for a wide range of biomarkers. It is designed for hospitals, clinics and laboratories to streamline diagnostic workflows.
2. What detection methodology does the Getein 1600 use?
It uses lateral flow chromatography with immunofluorescence detection for quantitative measurement of targets on disposable assay strips.
3. What sample types can the analyzer accept?
Supported sample types include whole blood, serum, plasma, urine and fingertip blood (capillary).
4. Which tests and biomarkers are available on the Getein 1600?
The analyzer supports multiple panels including Cardiac markers (hs-cTnI, cTnI, BNP, NT-proBNP, CK-MB, Myo, H-FABP), Coagulation (D-Dimer), Inflammation (hs-CRP, PCT, SAA, IL-6), Renal (CysC, mAlb, NGAL), Diabetes (HbA1c), Thyroid (TSH, T3, T4, fT3, fT4), Tumor markers (tPSA, fPSA, AFP, CEA), Fertility/Reproduction (HCG+β, FSH, LH, AMH, Progesterone, Testosterone) and others (Ferritin, Total IgE, Anti-HCV, Anti-TP).
5. What is the throughput and turnaround capability?
The system can run up to 48 samples per run and achieve up to 150 tests per hour with continuous loading. Exact turnaround per assay varies by test; consult the assay insert for per-test time.
6. How are results displayed and printed?
Results are shown on a 10.4-inch LCD touch screen (1024×768) and can be printed using the internal thermal printer. Results are also stored electronically for export or network transfer.
7. How much data can the Getein 1600 store and how is data transferred?
The analyzer stores over 500,000 results. Data connectivity options include LIS/HIS integration, Wi‑Fi/4G data transmission and network printer support for electronic transfer. Check with your local distributor for specific export formats and integration services.
8. Does the instrument prevent cross-contamination?
Yes. The system uses disposable filter tips and automated sample handling to minimize cross-contamination risk.
9. How are reagents and consumables managed?
Reagents are barcode-recognized for reagent ID and traceability. Use validated Getein reagents and consumables; reagent lot tracking and expiry are managed via the software. Replace disposable tips and reagent cartridges per the instructions for use.
10. What calibration and quality control features are available?
The Getein 1600 supports automatic calibration and comprehensive QC management. The software allows routine QC runs, result flagging and documented SOP workflows. Performance characteristics and recommended QC schedules are provided in the assay manuals.
11. Is there a STAT or emergency testing mode?
Yes. The analyzer offers diversified test modes including random, batch and STAT to prioritize urgent samples while maintaining continuous loading capability.
12. What are the physical dimensions and weight of the analyzer?
Dimensions are 639 mm (D) × 562 mm (W) × 728 mm (H). The unit weighs approximately 45 kg. Allow additional clearance for service access and ventilation.
13. What environmental and power requirements are needed for installation?
Specific environmental (temperature/humidity) and power requirements are provided in the installation manual and should be followed for optimal performance. Contact your supplier or consult the user manual for detailed site preparation and electrical specifications.
14. What training, maintenance and technical support are available?
Getein and authorized distributors typically provide installation, user training, preventive maintenance guidance and technical support. Service contracts and on-site training options vary by region; contact your local Getein representative for details.
15. Is the Getein 1600 certified for clinical use in my country?
Regulatory approvals vary by market. Please consult Getein Biotech or your local distributor for documentation on certifications (CE, local regulatory clearances, etc.) applicable in your country and for details on clinical use compliance.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading