B21, China Town Mall, Midrand
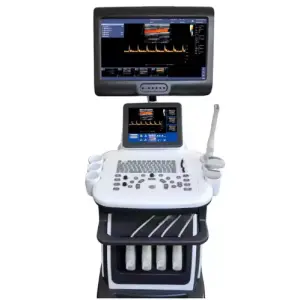
Ophthalmic A/B-Scan Ultrasound Biometer Portable Ophthalmic AB Scanner Oral Therapy Equipments & Accessories
- Section : Medical Supplies
- Category : Oral Therapy Equipments
- SKU : 1601415617580

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 06 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
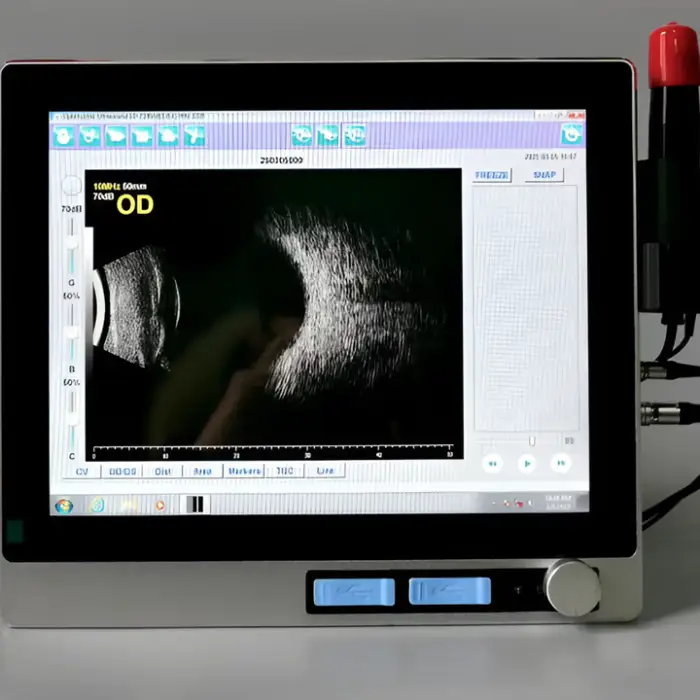
1. What is the Ophthalmic A/B-Scan Ultrasound Biometer?
The Ophthalmic A/B-Scan Ultrasound Biometer (Model A/B-Scan, brand New Vision) is a portable medical device that performs A-scan (axial length and biometric measurements) and B-scan (two-dimensional cross-sectional imaging) ultrasound for ophthalmic diagnosis and treatment planning.
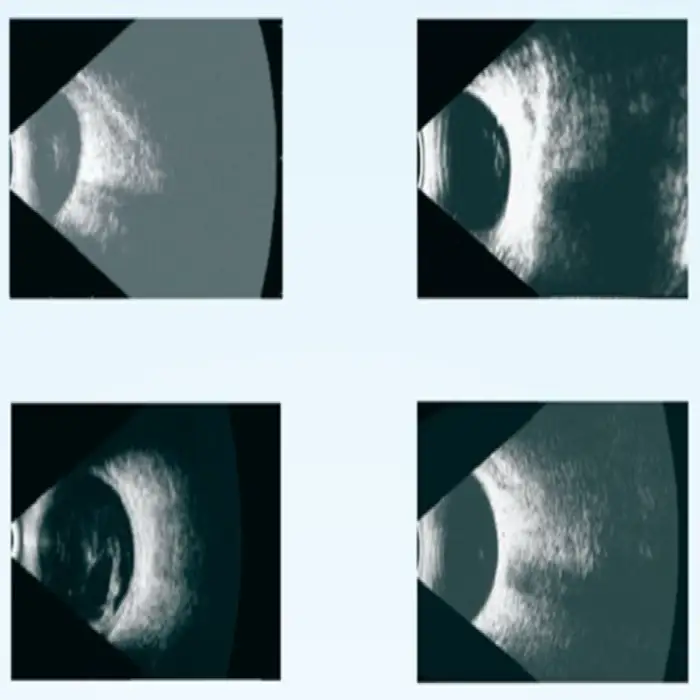
2. What is the difference between A-scan and B-scan modes?
A-scan provides one-dimensional axial measurements such as axial length used in IOL calculations. B-scan produces 2D cross-sectional images of the eye, useful for visualizing posterior segment structures when direct visualization is difficult.
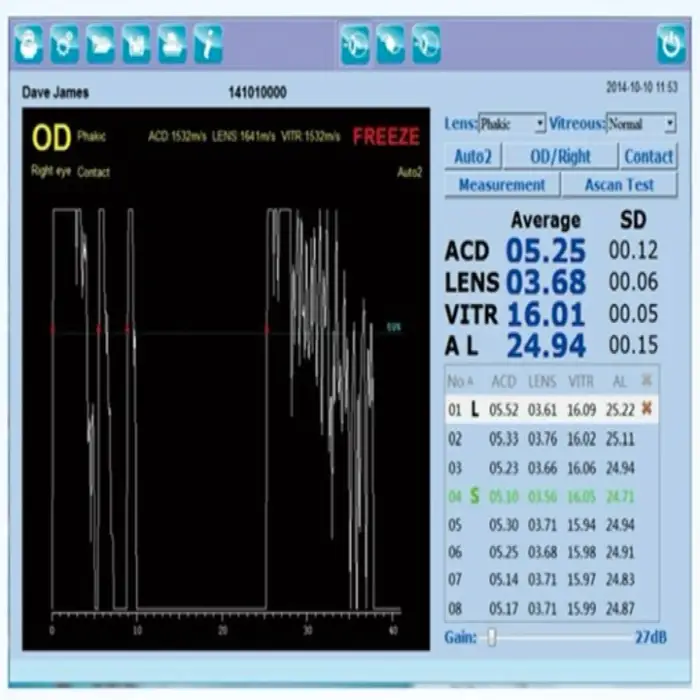
3. Is this device suitable for intraocular lens (IOL) power calculations?
The A-scan function measures axial length, which is a key input for IOL power calculations. Whether IOL power calculation software is included depends on the specific configuration—confirm with the supplier if built-in IOL formulas and calculation workflows are provided.
4. What are the power requirements and portability details?
The device accepts 110V/220V electric power. It is designed to be portable for transport between clinical locations. Single gross weight is listed as 20.000 KG and package dimensions are 35 x 45 x 50 cm.
5. What is the display resolution and image quality?
The unit features a high-resolution display with 1280 x 800 pixel resolution to provide clear imaging for both A- and B-scan modes.
6. What certifications and device classification does it have?
The product carries CE quality certification and is listed as a Class I medical device.
7. What accessories and probes come with the unit?
Standard configurations often include A-scan and B-scan probes, probe cables, power cord, and basic accessories; exact contents vary by supplier. Confirm the supplied accessories and optional extras (e.g., printer, carrying case, disposable probe covers) with the vendor.
8. How should the probe and device be cleaned and disinfected?
Follow the manufacturer's cleaning and disinfection instructions. In general, use manufacturer-approved disinfectant wipes or solutions, avoid immersing electronic components, and do not autoclave probes unless explicitly stated. Use single-use probe covers when appropriate.
9. How is the device maintained and calibrated?
Routine maintenance and periodic calibration are recommended to ensure measurement accuracy. Follow the user manual for cleaning and inspection schedules. Calibration or service should be performed by qualified technicians or authorized service centers; online technical support is available from the manufacturer.
10. What is the stated shelf life and warranty information?
The specification lists a shelf life of 1 year. Warranty terms are not specified in the listing—contact the seller or manufacturer for warranty duration, support packages, and return policies.
11. Can the device export images and data to electronic medical records (EMR)?
Connectivity options are not detailed in the provided specifications. Many modern units offer USB, network, or DICOM/CSV export capabilities; verify supported data export formats and connectivity options with the supplier.
12. Is this device safe for patient use and are there any contraindications?
The device is a CE-marked Class I medical device designed for ophthalmic use. Standard ultrasound safety practices should be followed. Contraindications or precautions (e.g., suspected open globe injury) should be reviewed in the device manual and clinical protocols—consult the device manual and clinical guidelines before use.
13. Do operators require special training to use the unit?
Yes. Proper operation and interpretation of A- and B-scan requires training in ophthalmic ultrasound. Manufacturers or distributors typically provide user manuals, training resources, or online technical support to assist new users.
14. Where is this device typically used?
It is intended for ophthalmology clinics and practices for eye examinations, aiding in diagnosis of cataracts, posterior segment conditions, and other ocular pathologies where axial measurements or ultrasound imaging are required.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading