B21, China Town Mall, Midrand
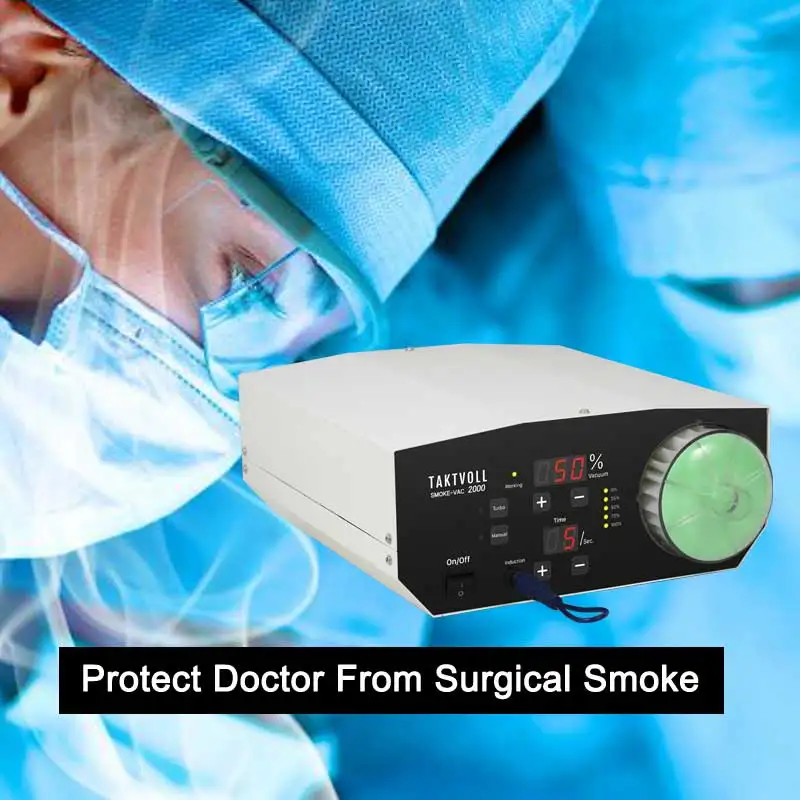
Surgical smoke evacuation
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1600281736972

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 05 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
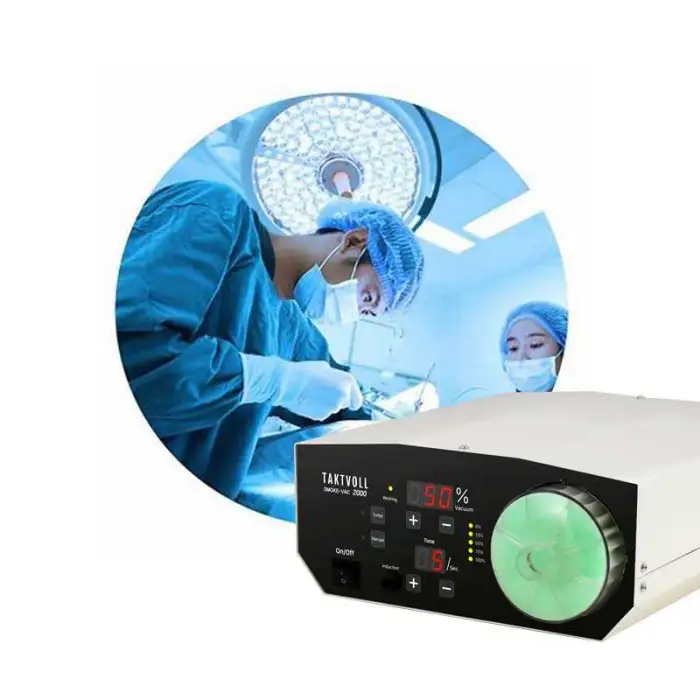
1. What is the Smoke-Vac 2000 and what does it do?
The Smoke-Vac 2000 is a surgical smoke evacuation device designed to remove and filter surgical smoke generated during procedures (for example LEEP, microwave treatments, and CO2 laser operations). It reduces airborne particulate matter, cellular debris, and chemical byproducts to improve air quality in the operating field.
2. How effective is the Smoke-Vac 2000 at removing harmful particles and viruses?
The system uses high-efficiency filtration and a 200W suction motor to capture particulate debris and many airborne contaminants. The product is described as filtering harmful particles, including biological material and viruses such as HPV and HIV. While the device reduces airborne hazards, it should be used alongside standard infection-control practices and personal protective equipment; no single device can guarantee 100% elimination of risk.
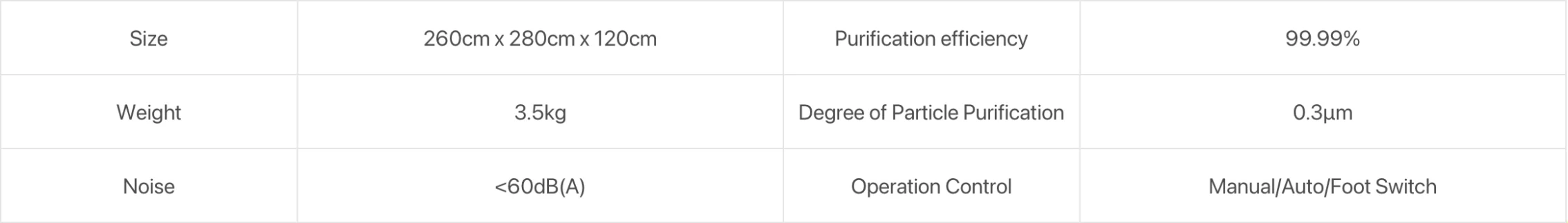
3. What are the key technical specifications?
Key specifications provided: 200W motor power, filter life up to 12 hours of use, manual or foot-pedal operation, LED real-time power/status display, intelligent filter monitoring and error alerts, and a compact design for shelf or cart placement.
4. How do I know when the filter needs to be replaced?
The Smoke-Vac 2000 includes intelligent monitoring that tracks filter service life and connection status. The system will issue an alert code when the filter is near or at the end of its service life. The nominal filter life is up to 12 hours; actual life depends on procedure type and smoke load.
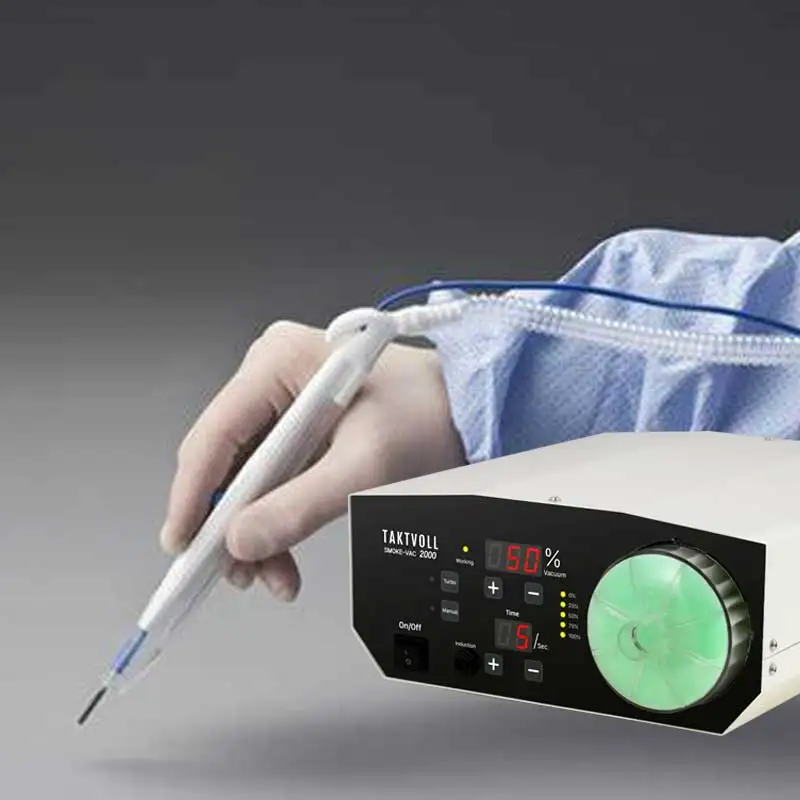
5. Can the device be operated hands-free?
Yes. The Smoke-Vac 2000 supports both manual control and a foot-pedal switch for hands-free operation during procedures.
6. Is the device noisy during operation?
No — the Smoke-Vac 2000 is designed for quiet operation and is described as operating silently even at high flow rates to minimize disruption in the surgical environment.
7. Where can the Smoke-Vac 2000 be installed or positioned in the OR?
Its compact design allows placement on a shelf or integration into a procedure cart alongside other equipment (for example an electrosurgical generator). Ensure adequate ventilation and access to power and controls per your facility’s policies.
8. What maintenance is required?
Routine maintenance includes replacing filters according to the device alerts or after 12 hours of use, inspecting hoses and connections, wiping external surfaces with appropriate disinfectant, and following manufacturer instructions for periodic checks. Always decontaminate surfaces and handle used filters as biohazardous waste.
9. How should used filters be disposed of?
Used filters may contain biological and hazardous material and should be disposed of in accordance with local regulations and hospital biohazard waste procedures. Follow manufacturer disposal guidance and institutional infection-control policies.
10. Does the Smoke-Vac 2000 integrate with other surgical equipment?
Yes. The compact form factor allows placement near or on the same cart as other surgical devices such as electrosurgical generators. Confirm compatibility of accessories (hoses, booms, laryngoscopes, etc.) and follow setup instructions for safe integration.
11. Are there any certifications or standards the Smoke-Vac 2000 meets?
Certification details are not provided in this description. For information about regulatory approvals, safety standards, or certifications (for example CE, FDA, ISO), contact the manufacturer or vendor and request documentation before purchase or use.
12. Does using the Smoke-Vac 2000 replace the need for PPE?
No. Smoke evacuation reduces airborne contaminants but does not replace standard PPE and infection-control practices. Continue to use gloves, masks/respirators, eye protection, and other PPE as required by local protocols.
13. What training is required to operate the device?
Operators should receive training on device setup, filter replacement, interpreting LED displays and alert codes, proper suction positioning, and safe disposal of contaminated filters. Follow the manufacturer’s user manual and your institution’s device training requirements.
14. Who should I contact for technical support, replacement filters, or warranty information?
Contact your Smoke-Vac 2000 vendor or the device manufacturer for technical support, genuine replacement filters and accessories, and warranty or service contract details. Keep product serial numbers and purchase documentation available when contacting support.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading