B21, China Town Mall, Midrand
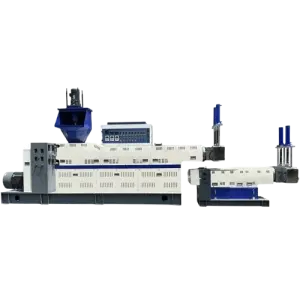
Supply Disposable Oxygen Catheter Injection Molding Machine Medical Equipments Making Machine
- Section : Machinery
- Category : Plastic & Rubber Processing Machinery
- SKU : 1600636315922

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 23 Mar, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Supply Disposable Oxygen Catheter Injection Molding Machine (JY-250ST) used for?
The JY-250ST is a precision plastic injection molding machine designed to produce small, high-accuracy plastic parts — including components for disposable medical devices such as oxygen catheters — when paired with the appropriate molds and medical-grade materials.
2. What are the key technical specifications of the JY-250ST?
Key specs include: clamping force 35 tons; platen size 540 × 380 mm; screw diameters available Φ26 / Φ28 / Φ30 mm; max shot weight 56 / 64 / 74 g (depending on screw); max shot rate 57 / 66 / 76 (depending on screw); screw stroke 115 mm; screw speed 0–205 rpm; nozzle contact force 4.45 tons; nozzle retraction 170 mm; three temperature control zones; total power 6.3 kW; machine weight ≈0.92 ton; dimensions 1.55 × 0.93 × 2.45 m.
3. What utilities does the machine require?
Utilities include: hydraulic system max pressure 140 kg/cm², pump output 34.5 L/min, oil reservoir 100 L, cooling water consumption ~400–600 L/hr, pump motor 3.75 kW (5 HP), barrel heating 2.4 kW. Electrical supply voltage and phase should be confirmed with the supplier prior to installation.
4. Which plastic materials can the machine process?
The machine can process a wide range of thermoplastics commonly used for medical components (subject to material temperature and molding parameters), such as PP, PE, PVC, ABS and engineering plastics. For medical-grade polymers, verify material compatibility, molding parameters and regulatory requirements.
5. What mold sizes and configurations does it support?
The platen area is 540 × 380 mm and the tie-bar spacing is 375 × 205 mm. Minimum mold height is shown as 158 / 58 mm (depending on configuration) and maximum opening mold distance is 338 / 238 mm. Confirm your mold’s footprint and height against these dimensions before ordering.
6. Is the JY-250ST suitable for producing disposable oxygen catheter components?
Yes — the machine is suitable for producing small, precision medical parts such as components for disposable oxygen catheters, provided you use appropriate medical-grade materials, mold design, process controls, and follow applicable regulatory and cleanroom requirements.
7. What safety and control features are included?
Notable features include a low-pressure closed die assembly for die safety, fixed bottom / loose top mold arrangement with ejector, straight screw ejected assembly for consistent performance, adjustable process parameters, and an LED display for machine control. Standard safety guarding and interlocks should be installed per local regulations.
8. How do I estimate cycle time and production output?
Cycle time depends on part geometry, material, mold design and cooling requirements. Small medical parts commonly run short cycles (often in the range of several seconds to a minute), but exact estimates require part drawings and process details. Contact the supplier with your part and mold to get a production estimate.
9. What routine maintenance does the machine need?
Routine maintenance includes: checking and replacing hydraulic oil and filters; inspecting heater bands, thermocouples, nozzle and barrel; monitoring screw/barrel wear; cleaning cooling circuits and water filters; verifying ejector and platen condition; and following the manufacturer's recommended preventive maintenance schedule.
10. What are the machine dimensions and shipping information?
Machine dimensions are approximately 1.55 m (L) × 0.93 m (W) × 2.45 m (H) and machine weight ≈0.92 ton. Shipping weight is about 1.12 tons and shipping measurements are roughly 1.55 × 1.14 × 2.32 m. Confirm final packing dimensions with your supplier for logistics planning.
11. What installation requirements should I prepare for?
Prepare a level, sturdy floor capable of supporting the machine weight and vibration; ensure proper electrical supply (confirm voltage/phase), cooling water and hydraulic connections; allocate space for mold handling and part collection; and arrange for professional installation and commissioning by authorized technicians.
12. Is training and documentation provided?
Most suppliers provide operator manuals and can offer on-site or remote training for machine operation, maintenance and process setup. Confirm available training packages and documentation with your sales contact.
13. Are spare parts, service and warranty available?
Spare parts, maintenance service and warranty terms vary by supplier. Contact the manufacturer or authorized distributor for information on warranty coverage, recommended spare parts kits and available service contracts.
14. Can the machine be customized for specific medical part requirements?
Yes — the JY-250ST supports adjustable process parameters and multiple screw diameter options. Additional customizations (e.g., servo drives, different ejector strengths, mold interfaces or automation add-ons) may be available — discuss specific requirements with the supplier.
15. What regulatory and cleanliness considerations apply when producing medical disposables?
Manufacturing medical disposables requires control of materials, process validation, traceability and often cleanroom/controlled environment production. The machine itself is a production tool; achieving regulatory compliance (e.g., ISO 13485, FDA) depends on process controls, validated molds, material certifications and production environment. Work with your quality/regulatory team and supplier to implement compliant processes.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals