B21, China Town Mall, Midrand
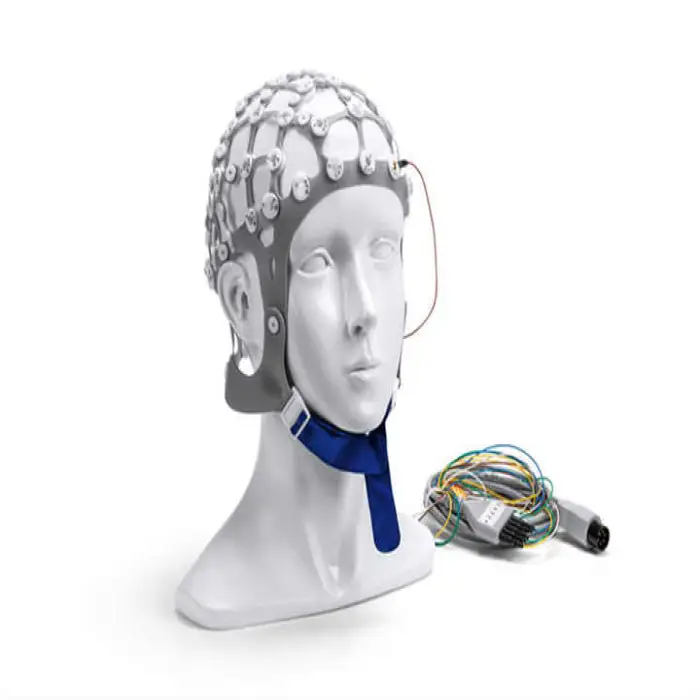
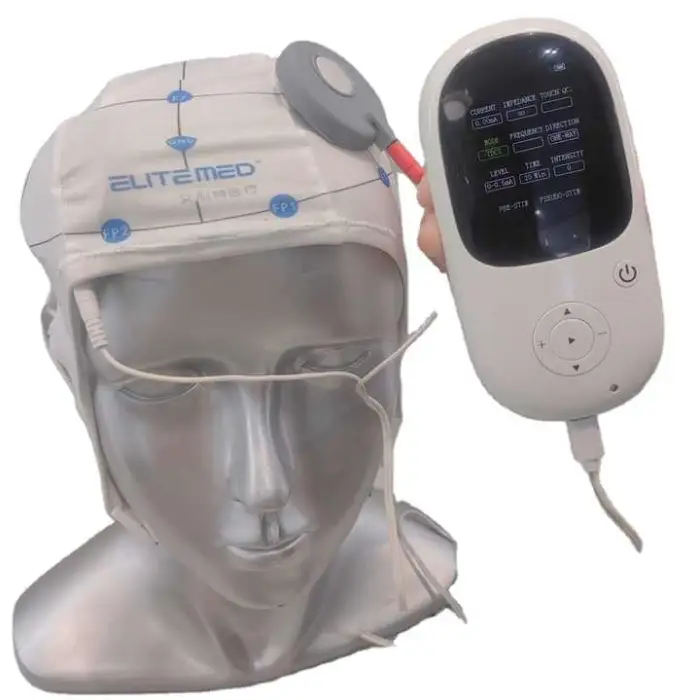
Strokes Stimulator TDCS Transcranial Electrical Stimulation Therapy Post-Stroke Neurorehabilitation Machine
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 10000015713529

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 05 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Strokes Stimulator TDCS?
The Strokes Stimulator TDCS (Model E-TDCS02 by ELITEMED) is a transcranial direct current stimulation device designed to support post-stroke neurorehabilitation by delivering low‑intensity electrical stimulation to promote recovery as part of a rehabilitation program.
2. Who should operate this device?
This device should be used under the guidance of a qualified healthcare professional. Use at home is possible only with professional instruction and an established treatment plan.
3. What are the primary applications of this machine?
It is intended for post-stroke neurorehabilitation and for supporting brain-function enhancement through transcranial electrical stimulation in hospitals, rehabilitation centers, and supervised home settings.
4. What power source does the device use?
The Strokes Stimulator TDCS is electric and uses a plug-in power supply mode. It does not contain an internal battery.
5. What safety certifications and classification does the product have?
The product is ISO13485 certified and is classified as a Class II instrument, indicating it is subject to regulatory controls for safety and performance.
6. Are there any known contraindications or precautions?
Contraindications and precautions should be reviewed with a clinician. Generally, transcranial electrical stimulation is approached with caution in people with implanted electronic medical devices (e.g., pacemakers), seizure disorders, open cranial wounds, or pregnancy. Always consult a healthcare professional before use.
7. Does the device come with technical support?
Yes. Online technical support is available. Contact details and support procedures are provided in the product manual or via the ELITEMED support channels.
8. What materials is the device made from and how do I clean it?
The housing is made of durable ABS material. Clean the exterior with a soft cloth dampened with mild detergent; do not immerse the device in liquid or use abrasive cleaners. Follow the user manual for electrode and accessory cleaning instructions.
9. What accessories or electrodes are compatible with the unit?
Use electrodes and accessories recommended by ELITEMED or those specified in the user manual. If using third‑party electrodes, confirm compatibility and safe connection with the manufacturer or clinician before use.
10. Is special training required to use it?
Yes. Proper training in tDCS application, electrode placement, device settings, and monitoring is recommended for clinicians and caregivers to ensure safe and effective use.
11. How long is the shelf life and what are the package dimensions/weight?
The listed shelf life is 1 year. Single package dimensions are 46 x 51 x 37 cm and the single gross weight is approximately 9.000 kg.
12. Can this device replace conventional rehabilitation therapies?
No. The device is intended to support neurorehabilitation as an adjunct to conventional therapies (physical, occupational, speech therapy). Treatment plans should be determined by rehabilitation professionals.
13. What maintenance or inspections are recommended?
Perform routine visual inspection of the unit, power cord, connectors and electrodes before each use. Follow the maintenance schedule in the user manual. Seek professional servicing for any faults; do not attempt to repair internal components yourself.
14. What should I do if the device malfunctions or I have safety concerns?
Stop using the device immediately, disconnect it from power, and contact ELITEMED technical support or your supplier. Report any adverse events to your clinician and follow local medical device adverse event reporting procedures.
15. How do I obtain more detailed clinical or regulatory information?
For detailed clinical data, regulatory status in your country, or instructions for use, refer to the product manual and ELITEMED’s official documentation or contact their customer support.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading