B21, China Town Mall, Midrand
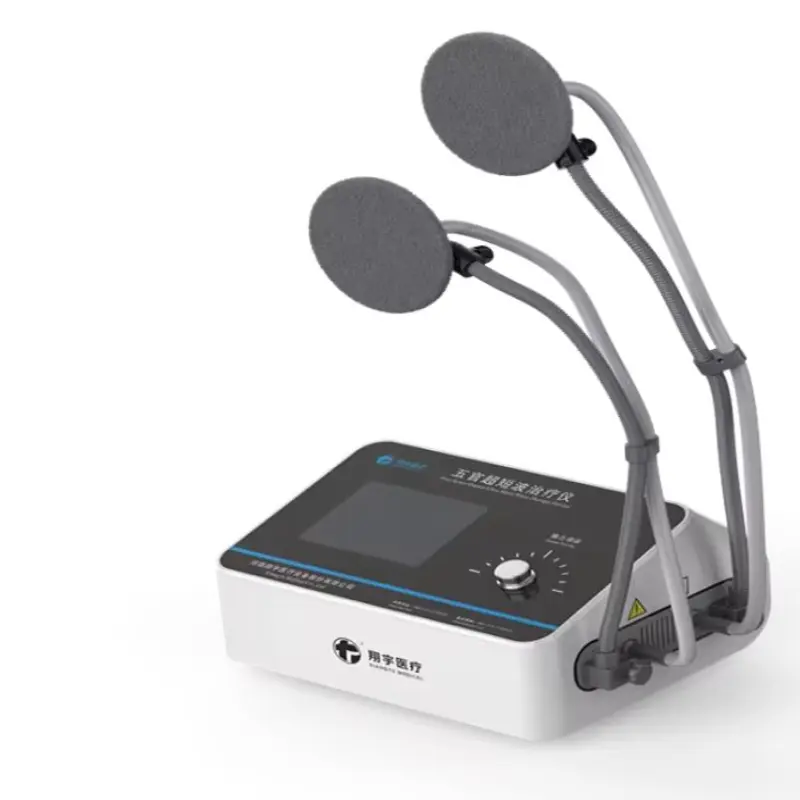
Rehabilitation Equipment Five Sense Organs Ultra Shortwave Therapy Device
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1601039266105

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 05 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Rehabilitation Equipment Five Sense Organs Ultra Shortwave Therapy Device?
The device (Model XY-WGCDB-I) is an ultra-shortwave therapeutic unit designed to provide targeted pain-relief and rehabilitation treatments for the five sense organs using a 40.68 MHz working frequency and adjustable output power.
2. What are the available output power settings?
The device offers four selectable output power levels: 20W, 30W, 40W, and 50W to tailor therapy intensity to patient needs.
3. What is the working frequency and why does it matter?
The unit operates at 40.68 MHz; this ultra-shortwave frequency is chosen to deliver efficient therapeutic energy for soft tissue and pain-relief applications when used according to professional guidelines.
4. What treatment time options does the device provide?
Preset treatment durations are available at 10, 15, 20, 25, and 30 minutes to accommodate different therapy protocols.
5. What are the power and electrical requirements?
The device is powered by 220 VAC with a rated input power of 180 VA; for other mains voltages or international configurations, please contact the supplier.
6. What are the device dimensions and is it suitable for small treatment rooms?
The compact dimensions are 455 mm x 360 mm x 235 mm (L/W/H), making it suitable for clinics, hospitals, and rehabilitation centers with limited space.
7. Who should operate this device?
It should be operated by trained medical or rehabilitation professionals familiar with ultra-shortwave therapy and patient safety protocols.
8. Are there any contraindications or precautions?
Contraindications typically include patients with implanted electronic devices (e.g., pacemakers), pregnancy (especially over the abdomen/pelvis), active bleeding, malignancy in the treatment area, or acute infection; always perform a clinical assessment and consult local guidelines before treatment.
9. Is the device certified for quality and safety?
The product is ISO 13485 certified, indicating compliance with international quality management standards for medical devices; additional regional approvals (e.g., CE, FDA) depend on local registration and are not specified here.
10. What maintenance and cleaning procedures are recommended?
Turn off and unplug the unit before cleaning, wipe external surfaces with a soft damp cloth and mild detergent, avoid liquid ingress into vents or controls, inspect cables and applicators regularly, and arrange professional servicing for internal checks or repairs.
11. What accessories or consumables are included and are extras available?
Standard delivery typically includes the main unit and required applicators; optional accessories or replacement parts may be available—contact the manufacturer or distributor for a complete list and pricing.
12. Can the device be customized through OEM/ODM services?
Yes, the manufacturer offers ODM and OEM customization options to meet specific clinical or branding requirements—contact sales to discuss configuration, labeling, or software adjustments.
13. What should I do if the device fails to power on or malfunctions?
First check the power connection, mains supply, and fuses; if the problem persists, stop using the device and contact authorized technical support or the supplier for troubleshooting and repair.
14. Is there a minimum order quantity (MOQ) and how can I purchase one?
The MOQ is 1 set. To purchase, contact the manufacturer or an authorized distributor for pricing, lead times, shipping options, and after-sales support.
15. Are there recommended clinical applications and training resources?
The device is intended for pain relief and rehabilitation in clinical settings (rehab centers, hospitals, clinics). Operators should receive training from qualified personnel or the manufacturer on safe use, treatment protocols, and contraindications before clinical application.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading