B21, China Town Mall, Midrand
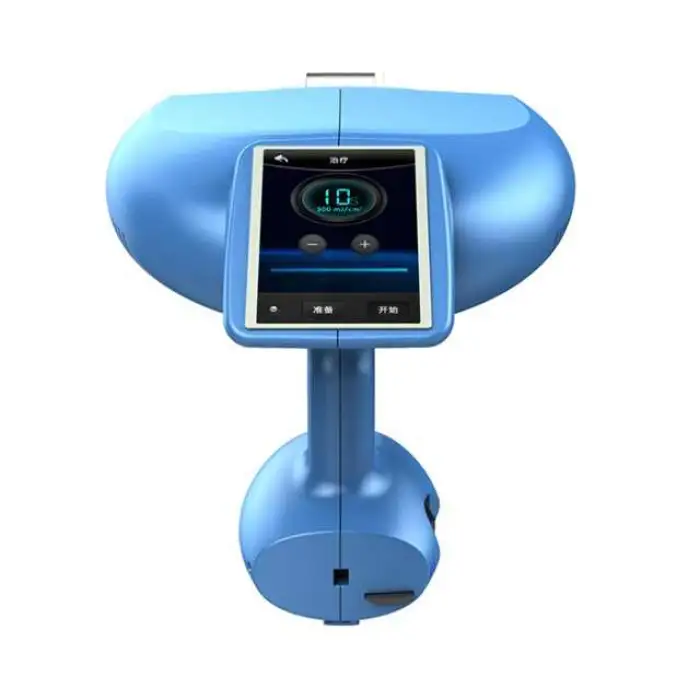
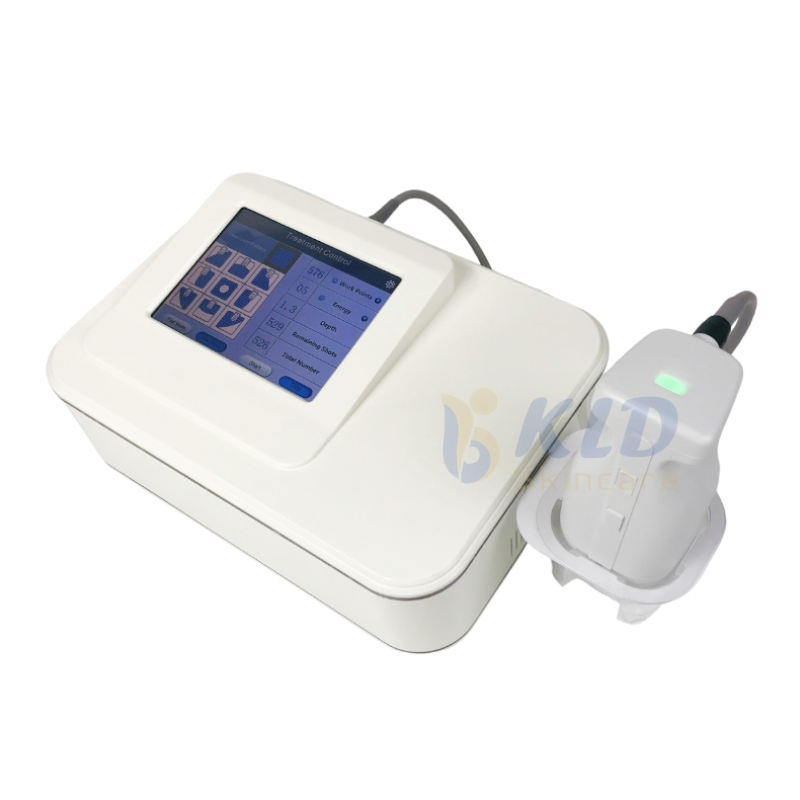
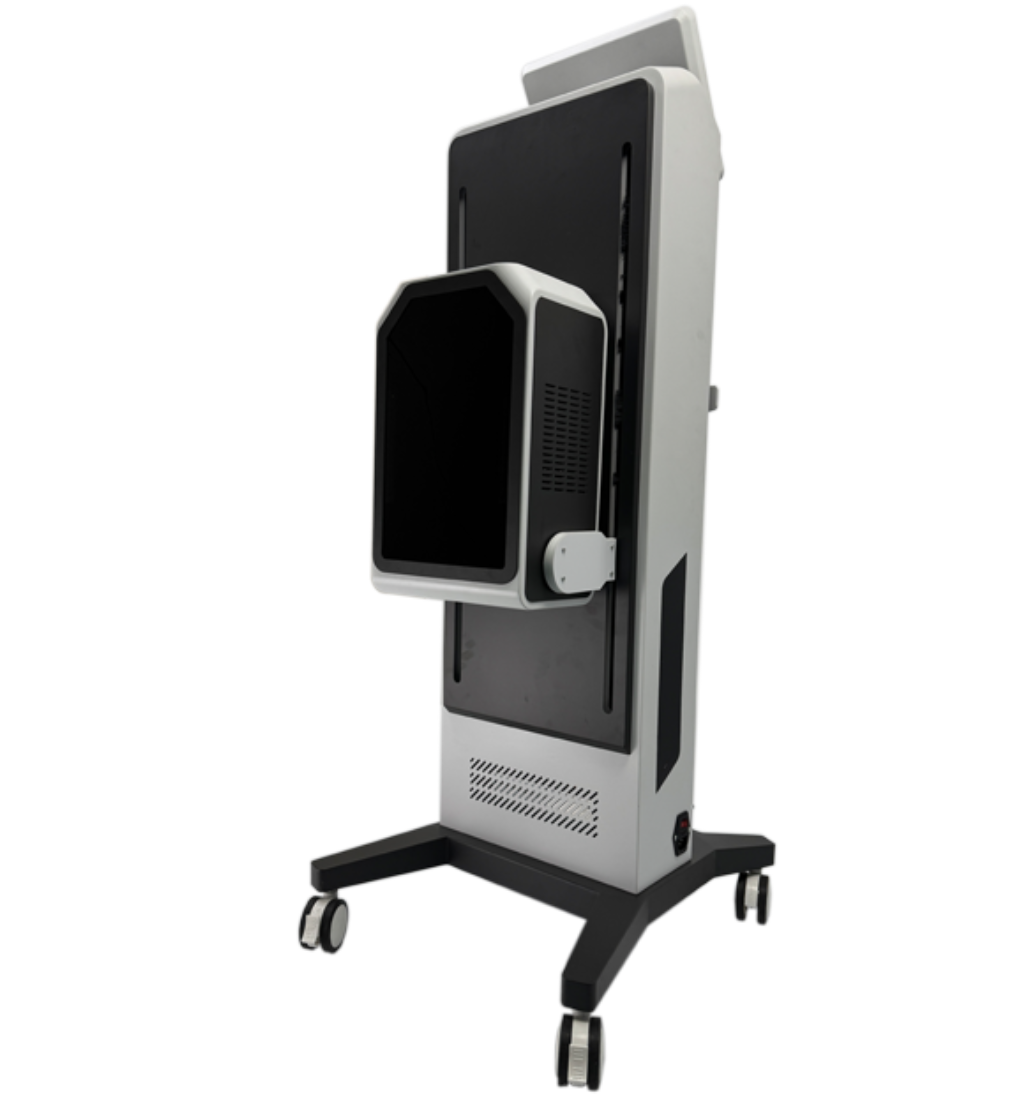
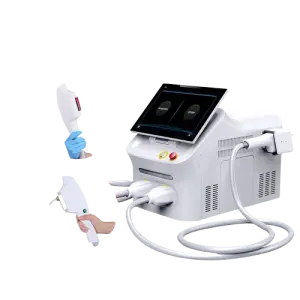
Portable for Psoriasis Lamp Led Uvb Phototherapy Home Use 308nm Excimer Psoriasis Vitiligo Therapy Equipment
- Section : Beauty & Personal Care
- Category : Hair Products
- SKU : 1601081459044

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 05 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What skin conditions does the Portable Psoriasis Lamp treat?
The device is designed primarily for treatment support of psoriasis and vitiligo using 308 nm excimer UVB light. It may also be used as part of other targeted phototherapy regimens as advised by a healthcare professional.
2. How does the 308 nm excimer light work?
The 308 nm excimer emits focused UVB light that targets affected skin areas. Phototherapy can reduce inflammation and abnormal cell activity in conditions like psoriasis and can be used to stimulate repigmentation in vitiligo under professional guidance.
3. Is this device safe for home use?
The lamp is marketed for home, beauty center, and clinic use and has a portable design. However, phototherapy carries risks; always follow the included user manual and treatment plan from a dermatologist before using at home.
4. Who should not use this phototherapy lamp?
Do not use the device if you have a known photosensitivity disorder, are taking photosensitizing medications, have active skin infections in the treatment area, or have a history of skin cancer without physician clearance. Pregnant or breastfeeding individuals should consult their healthcare provider first.
5. How often and how long should I use the lamp?
Treatment frequency and session length depend on the condition, skin type, and physician recommendations. Always follow a dermatologist-prescribed protocol; the device manual should also provide guidance on settings and safe exposure times.
6. What results can I expect and how long until I see improvement?
Response times vary by individual and condition severity. Some users may notice improvement after several sessions, while others require weeks of consistent treatment. Discuss realistic expectations and monitoring with your healthcare provider.
7. Are there any side effects?
Possible side effects include redness, mild burning, itching, blistering, or hyper-/hypopigmentation in treated areas. If you experience severe or persistent side effects, stop treatment and consult your healthcare provider.
8. What are the product specifications I should know?
Model: BDF001. Brand: Kexe. Material: ABS. Single package size: 43 x 35 x 45 cm. Single gross weight: 14.0 kg. It uses 308 nm excimer light and is described as a multi-function beauty machine suitable for home, beauty center, and clinic use.
9. Is the lamp certified and clinically validated?
Certification and clinical validation are not specified in the basic description. Request copies of relevant certifications (e.g., medical device marks, safety approvals) and clinical data from the supplier or manufacturer before purchase.
10. How portable is the device and what is included in the package?
The unit is described as portable and intended for travel and on-the-go treatments. Package contents are not fully detailed in the provided description—typical shipments may include the device, power adapter, and a user manual; confirm exact accessories with the seller.
11. How do I clean and maintain the lamp?
Unplug the device before cleaning. Wipe external surfaces with a soft, slightly damp cloth and a mild disinfectant recommended for electronic devices. Do not immerse the unit in water or use abrasive cleaners. Follow maintenance instructions in the user manual and contact the supplier for service or repairs.
12. Can I customize branding or labels on the device?
Yes. OEM/ODM and customizable labels are listed as acceptable. Contact the manufacturer or supplier to discuss minimum order quantities, artwork requirements, lead times, and additional costs.
13. What warranty or guarantee comes with the product?
The product description lists 'Guarantee Type: Multi-Function Beauty Equipment,' but specific warranty terms are not provided. Warranty coverage and duration typically depend on the seller—confirm warranty details, warranty start date, and claim process before purchasing.
14. Where can this device be used professionally?
The lamp is intended for use at home, in beauty centers, and in professional clinics. When used professionally, treatments should follow clinic protocols and be supervised by trained personnel or clinicians as appropriate.
15. How do I obtain replacement parts or technical support?
Contact the supplier or manufacturer (brand Kexe) for replacement parts (for example, LED modules or power adapters), technical support, and service. Keep your purchase information and serial/model number (BDF001) handy when requesting assistance.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading