B21, China Town Mall, Midrand
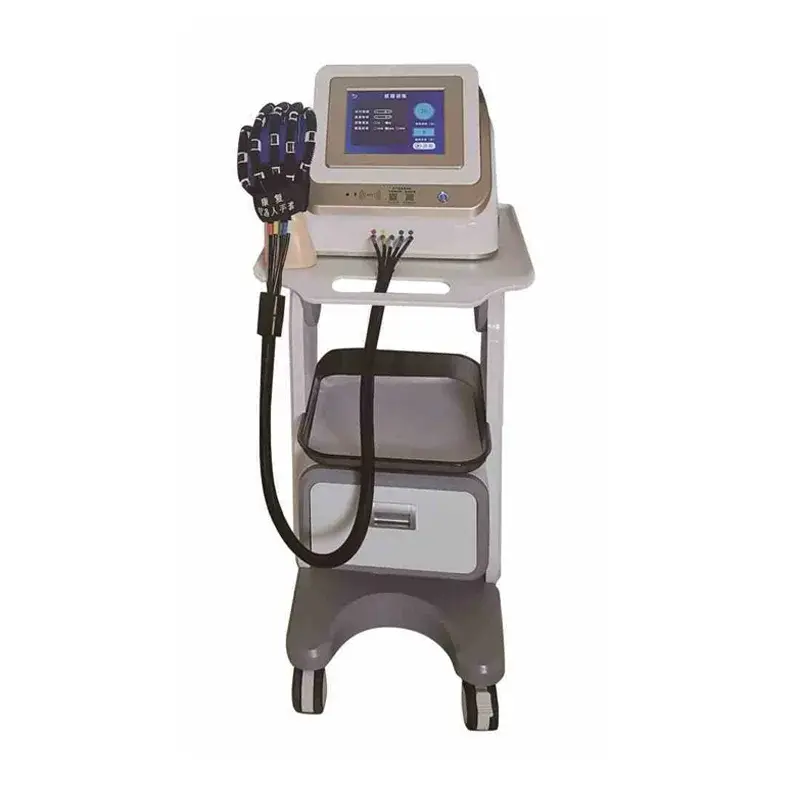
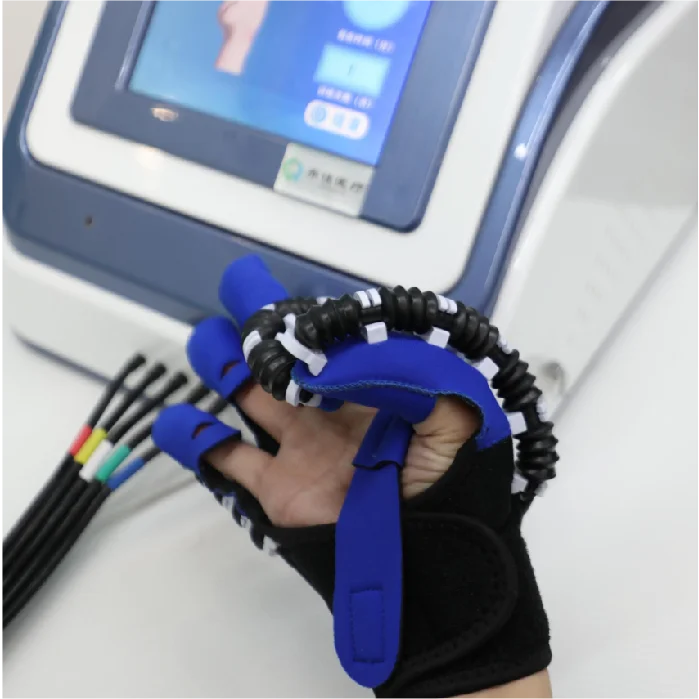
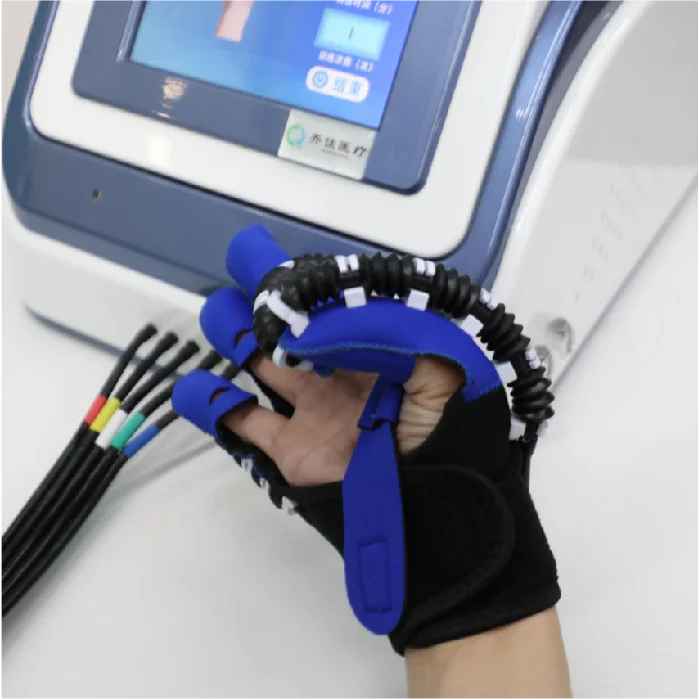
Pneumatic Joint Intelligent Rehabilitation System – HM-SK-8000 Model
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1601009880738

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 05 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Pneumatic Joint Intelligent Rehabilitation System – HM-SK-8000?
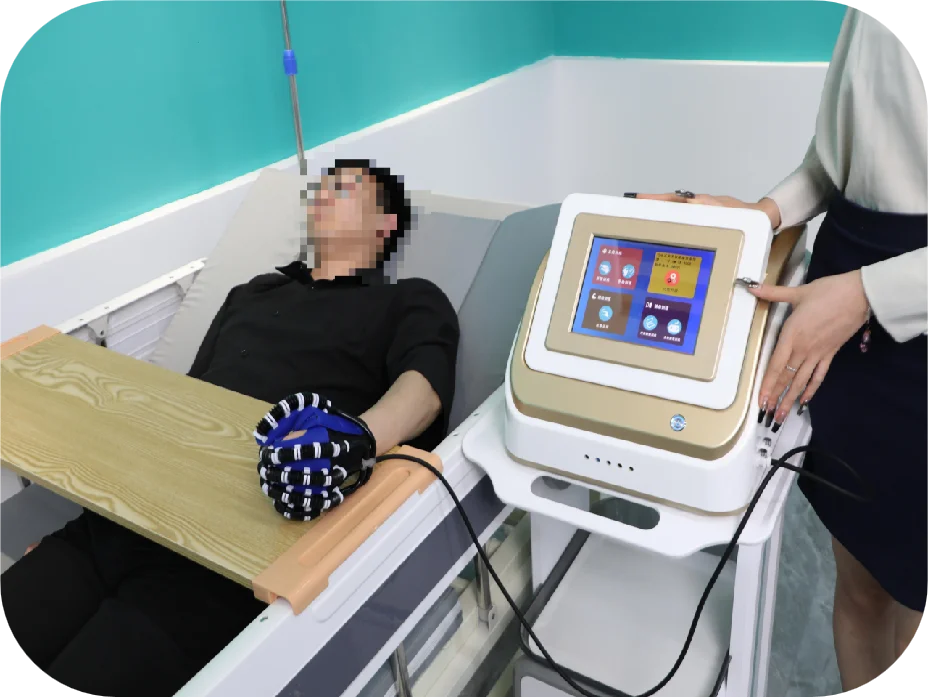
The HM-SK-8000 by Qijia is a Class II medical rehabilitation device that uses advanced pneumatic technology to assist recovery of hand function through controlled active and passive joint training. It is intended for use in rehabilitation centers and bedside settings.
2. What clinical problems or patients is the HM-SK-8000 intended to help?
The system is designed to support hand-function rehabilitation for patients recovering from stroke, orthopedic hand injuries, post-surgical recovery, neurological disorders, and other conditions affecting finger, wrist and hand mobility. Clinical suitability should be determined by a licensed healthcare professional.
3. What training modes does the device support?
The HM-SK-8000 supports passive training, master–slave (assistive) training, active training, auxiliary relaxation training, five-finger, single-finger, opposite-finger, multi-finger and mirror training modes to address a wide range of therapeutic goals.
4. How does the pneumatic technology work and what are its benefits?
The system uses pneumatic pressure to drive joint movements, providing smooth, controllable, and adjustable assistance or resistance. Benefits include gentle motion control, high repeatability, adjustable intensity and improved patient comfort compared with purely mechanical drives.
5. What evaluation and training content is included?
The device includes 52 evaluation scales (such as flexibility and coordination assessments), 32 interactive games and over 100 training methods that combine human–computer interaction and gamified tasks to increase patient engagement and track progress.
6. Can the HM-SK-8000 be used at a patient's bedside?
Yes. The system supports bedside passive training and portable hand-function rehabilitation setups for early intervention and convenient bedside rehabilitation.
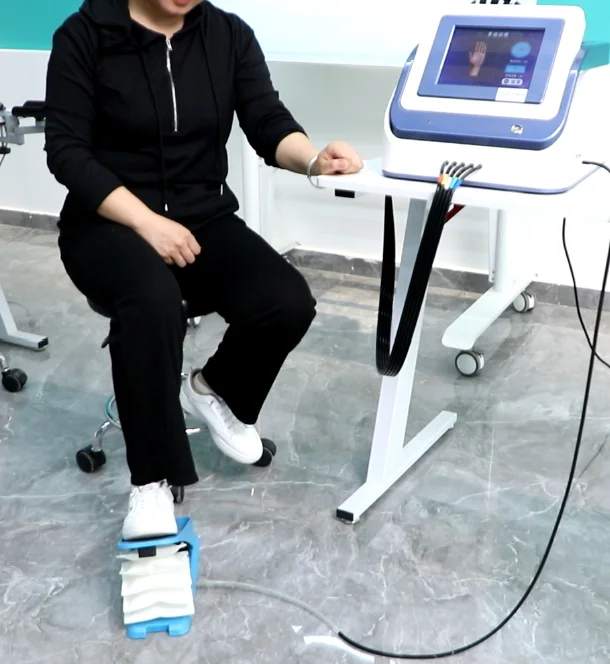
7. Does the system support lower-limb training or integration with other trainers?
Yes. The HM-SK-8000 can be connected with an ankle trainer to provide ankle up-and-down training, promoting lower-limb function and helping to prevent adverse spasm patterns during combined rehab.
8. What are the power and space requirements?
The unit operates on AC 220V, 50Hz power and requires approximately 0.5 square meters of floor space, making it compact and suitable for most rehabilitation facilities.
9. What safety certifications or regulatory class does the device have?
The HM-SK-8000 is classified as a Class II medical device, indicating it meets regulatory standards for safety and performance for clinical use. For specific certification documents or regional approvals, contact Qijia or your distributor.
10. How user-friendly is the interface and who operates it?
The system features an intuitive interface designed for rehabilitation professionals to set, monitor and adjust sessions easily. Clinician training is recommended to ensure safe and effective use.
11. What maintenance and cleaning procedures are required?
Routine maintenance includes visual inspections, cleaning external surfaces with mild disinfectant, checking pneumatic connections and filters, and following the manufacturer’s preventive maintenance schedule. Do not immerse electrical components; refer to the Qijia user manual for detailed instructions.
12. Are there any contraindications or precautions?
Contraindications and precautions include acute infections, open wounds at the treatment site, unstable fractures, severe joint instability, uncontrolled cardiovascular conditions, or any condition where passive joint movement may be harmful. Always perform a clinical assessment and follow device instructions. When in doubt, consult a physician.
13. How is patient progress tracked and can data be exported?
The HM-SK-8000 records evaluation scores, training parameters and performance in its software to track patient progress over time. For details on data export formats and integration with EMR systems, contact Qijia or your technical representative.
14. What accessories and training modules are available?
Available functions include bedside portable hand robots, single-finger and multi-finger interfaces, ankle trainer integration, and a library of game-based training modules. Contact Qijia or your distributor for a complete list of accessories and optional modules.
15. How do I get installation, training, warranty or service support?
Qijia or its authorized distributors provide professional installation, user training and after-sales service. Warranty terms, service packages and response times vary by region—contact Qijia or your local supplier for specific warranty details and support options.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading