B21, China Town Mall, Midrand
Physiotherapy Equipment Passive Rehabilitation Knee CPM Machine for Traction
- Section : Appliances
- Category : Household Appliances
- SKU : 1600704036214

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 05 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
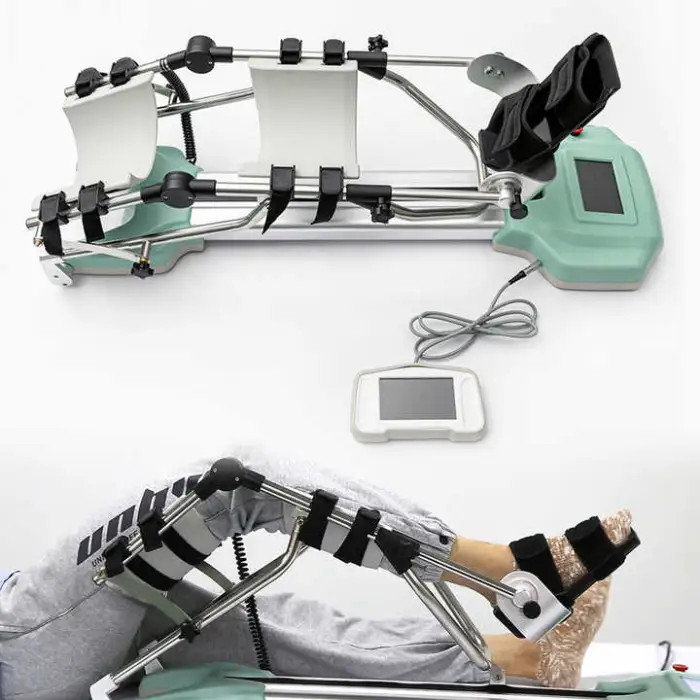
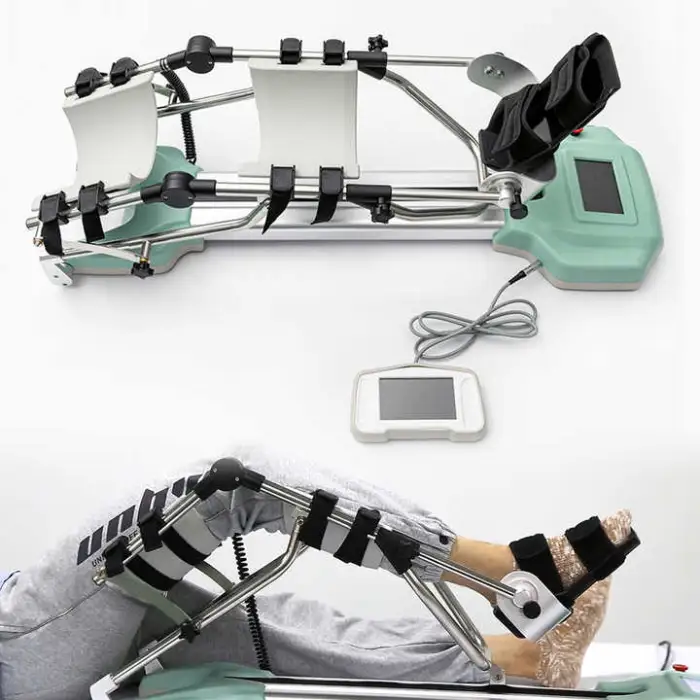
1. What is the Physiotherapy Equipment Passive Rehabilitation Knee CPM Machine for Traction?
A Continuous Passive Motion (CPM) device designed to move the knee joint gently and repeatedly to promote healing, reduce stiffness, and improve mobility after knee injury or surgery. It is suitable for use in rehabilitation centres and at home.
2. Who is this CPM machine intended for?
Patients recovering from knee surgery (e.g., ACL repair, total knee replacement), people with knee injuries or stiffness, and individuals prescribed passive range-of-motion therapy by a clinician. Use should follow the guidance of a physiotherapist or medical professional.
3. How does the CPM machine help with recovery?
By providing continuous passive motion to the knee, it helps maintain and progressively increase joint range of motion, reduces joint stiffness and edema, and can improve circulation to support healing of soft tissues and cartilage.
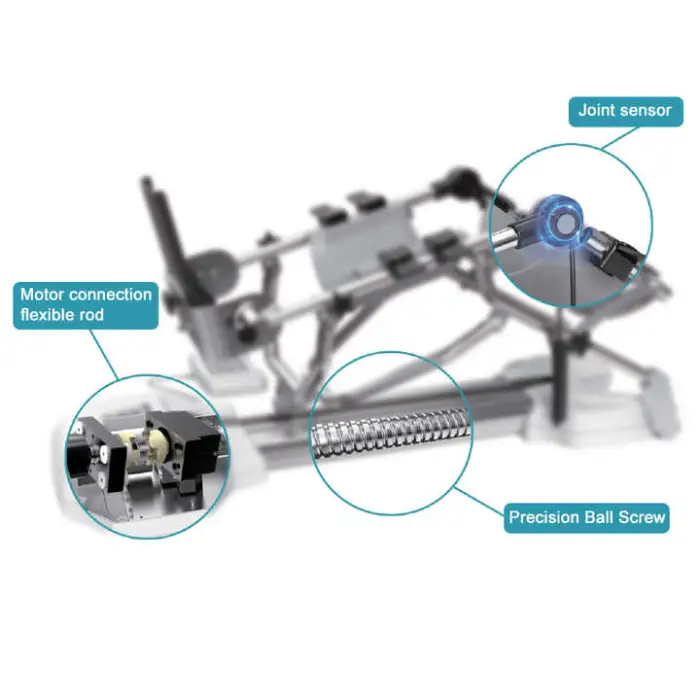
4. What are the main features of this model (CPM-B/YTK-G)?
Key features include a large-screen liquid display for easy control, durable ABS construction, service-free spare parts included, CE and ISO13485 certification, OEM availability, and design specifically for continuous passive motion of the knee.
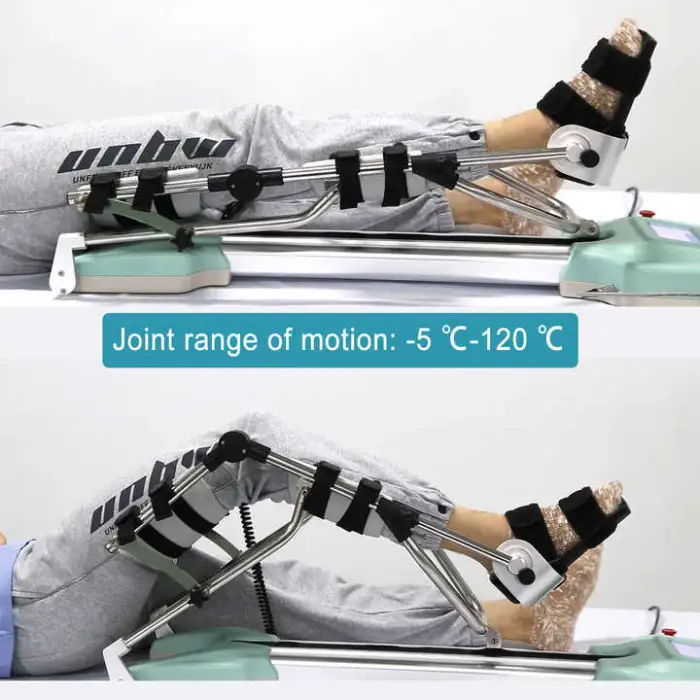
5. Is the range of motion and speed adjustable?
The device provides adjustable settings via its control interface (big screen display). Exact adjustment ranges and speed settings are provided in the user manual and can be personalized according to the treating clinician’s prescription.
6. Can the CPM machine be used at home?
Yes. It is suitable for home use as well as in rehabilitation centres. Home use should be done under the direction of a physiotherapist or surgeon and in accordance with the device manual.
7. What safety precautions or contraindications should I be aware of?
Contraindications include uncontrolled infection at the treatment site, unstable fractures, severe vascular compromise, or other conditions specified by a treating clinician. Always consult a physician or physiotherapist before use and follow the safety instructions in the manual.
8. How do I set up and start a treatment session?
General setup: place the machine on a stable surface, position the patient supine, align the limb with the device’s hinge axis, secure straps per instructions, power the unit and use the display to set prescribed range of motion and duration. Always follow the detailed step-by-step setup in the user manual and your clinician’s guidance.
9. What are the physical specifications (size, weight, packing)?
Single package size is 95 x 42 x 26 cm with a gross weight of 16.000 kg. The device is made of ABS material and comes packaged one piece per transparent bag and several pieces per carton as specified.
10. What certifications does the product have?
This model is CE certified and compliant with ISO13485, indicating conformity with relevant safety and quality standards for medical devices.
11. What maintenance and cleaning are required?
Wipe external surfaces with a soft cloth and a mild, non-abrasive disinfectant. Do not immerse the unit in liquid. Inspect straps, hinges and connectors regularly for wear. Follow the maintenance schedule and instructions in the user manual.
12. Are spare parts and service available?
The product description notes service-free spare parts are included. For additional parts, repairs or technical support, contact the supplier or manufacturer. OEM support is available if required.
13. What are the ordering, MOQ and supply capabilities?
Minimum order quantity (MOQ) is 1 piece. The supplier reports a supply ability of up to 10,000 pieces per month. OEM customization is available—contact the seller for lead times and customization details.
14. Where is the product shipped from and what are the shipping options?
Port of dispatch options include Shanghai, Ningbo, Guangdong and Shenzhen. Shipping terms and options should be arranged with the supplier and depend on destination, incoterms and carrier choice.
15. Who should I contact about warranty, technical specs (voltage, power) or clinical guidance?
Refer to the supplier or the product manual for warranty details and technical specifications (power/voltage). For clinical guidance on treatment duration, settings and contraindications, consult your surgeon or physiotherapist prior to use.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading