B21, China Town Mall, Midrand
Oral PDT Treatment Equipment Dental LIGHT PDT Photodynamic for Dentistry
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1600955518569

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 05 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
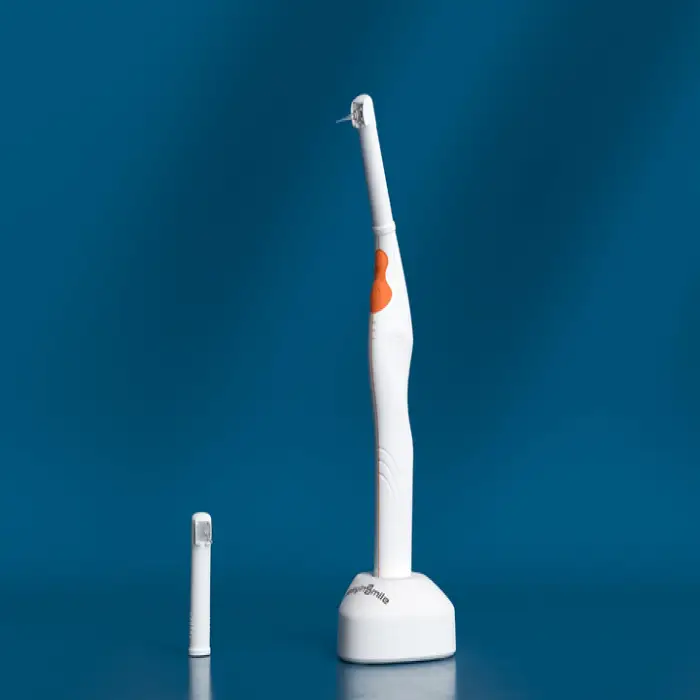
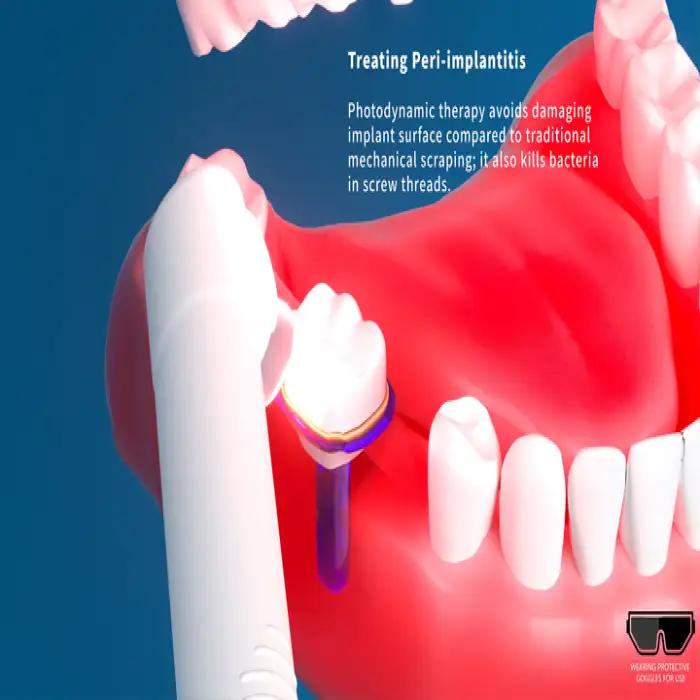
1. What is the Easyinsmile Oral PDT Treatment Equipment?
The Easyinsmile device is a dental photodynamic therapy (PDT) light system designed to assist dentists in treating oral infections, enhancing periodontal therapy, and supporting healing after procedures by using light-based photodynamic techniques.
2. How does photodynamic therapy (PDT) work in dentistry?
PDT combines a light source with a photosensitizing agent (when required) to activate a chemical reaction that reduces pathogenic bacteria and supports tissue healing. The device delivers controlled light energy to the treatment area according to clinical protocols.
3. What oral conditions can this equipment be used to treat?
Typical applications include treating oral infections, adjunctive periodontal therapy, improving post-procedural healing, and reducing bacterial load in the operatory as part of comprehensive dental care.
4. What is the device's regulatory classification and certifications?
The product is listed as a Class II dental instrument and carries CE certification for quality assurance. Always check local regulatory requirements before clinical use.
5. What is the power source and do I need special electrical arrangements?
The Easyinsmile device is electrically powered. Specific input voltage and plug type are provided in the user manual or by the supplier—verify compatibility with your clinic mains power and any regional adapters required.
6. Is special training required to operate the device?
Yes. Dentists and dental staff should receive training on PDT principles, the device's user instructions, patient selection, and safety procedures. Follow manufacturer training materials or supplier-provided training.
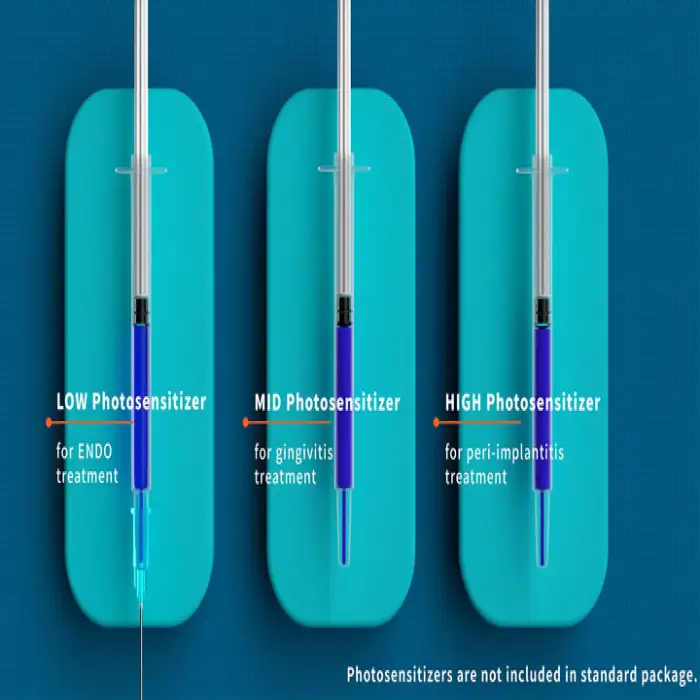
7. Does PDT always require a photosensitizing agent?
Some PDT protocols use topical photosensitizers (e.g., dyes), while other protocols may rely on endogenous chromophores or specific wavelengths. Use the treatment protocols recommended by the manufacturer or clinical guidelines.
8. What safety precautions should be observed during treatment?
Use appropriate eye protection for patient and staff, avoid direct viewing of the light source, follow contraindications (e.g., known photosensitivity, certain medications), and adhere to the manufacturer’s safety and clinical guidelines.
9. Are there any contraindications or patient groups to avoid?
Caution is generally advised for patients with known photosensitivity disorders, those taking photosensitizing medications, pregnant patients, or patients with uncontrolled systemic conditions. Always screen patients and consult the device manual or manufacturer for specific contraindications.
10. How should I clean and maintain the device?
Follow the manufacturer’s maintenance schedule. In general, wipe external surfaces with approved disinfectants, avoid immersing the device in liquids, inspect cables and connectors regularly, and replace damaged parts promptly. Refer to the user manual for detailed cleaning and maintenance instructions.
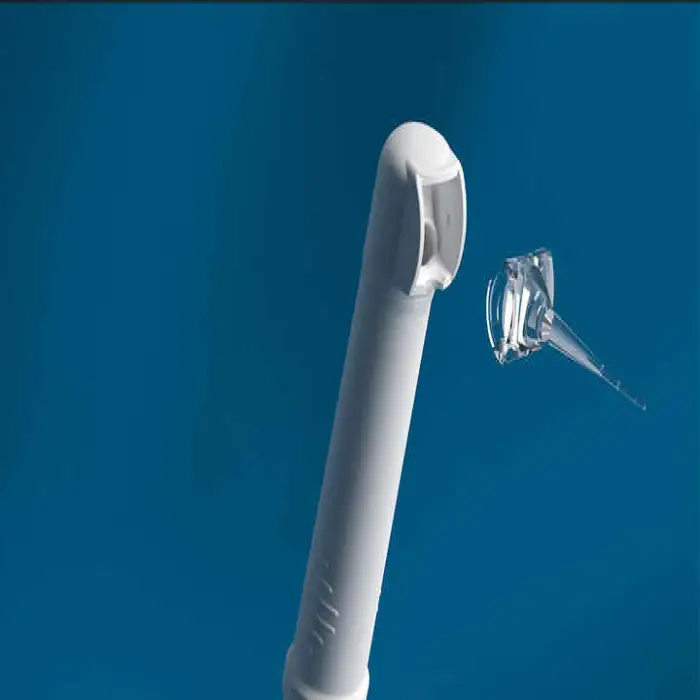
11. What parts of the device require sterilization before use?
Contact components or handpieces that contact mucous membranes must be cleaned and sterilized according to the manufacturer’s instructions. If disposable or single-use accessories are provided, use them as directed to prevent cross-contamination.
12. What is the product shelf life and how should it be stored?
The device has a stated shelf life of 3 years. Store it in a dry, temperature-controlled environment per the storage conditions in the product documentation and keep it in original packaging when possible.
13. What accessories, consumables, or replacement parts are needed?
Standard practice may require consumables such as photosensitizer solutions, disposable tips, protective goggles, or replacement bulbs/LED modules. The product is sold as a single item; contact your supplier or the manufacturer for available accessories and consumables.
14. Is there a warranty and where can I get service or repairs?
Warranty and service terms are not specified here. Contact the seller or manufacturer for warranty details, authorized service centers, and repair procedures. Use authorized technicians for servicing to maintain certification and safety.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading