B21, China Town Mall, Midrand
MT 100V Pro Electric Surgical Knife for Gynecology Surgery
- Section : Medical Supplies
- Category : Professional Medical Devices
- SKU : 1601012520506

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 05 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the MT 100V Pro Electric Surgical Knife?
The MT 100V Pro is a 100W high-frequency electrosurgical unit (monopolar cautery) designed primarily for gynecology surgery. It provides incision and coagulation functions in a compact, lightweight housing and is intended for use by trained surgical professionals.
2. Which surgical procedures is this device intended for?
It is specifically engineered for gynecological procedures but is also suitable for other delicate surgeries (e.g., general surgery, urology, oncology, orthopedics) where high-precision cutting and coagulation are required.
3. What are the key technical specifications?
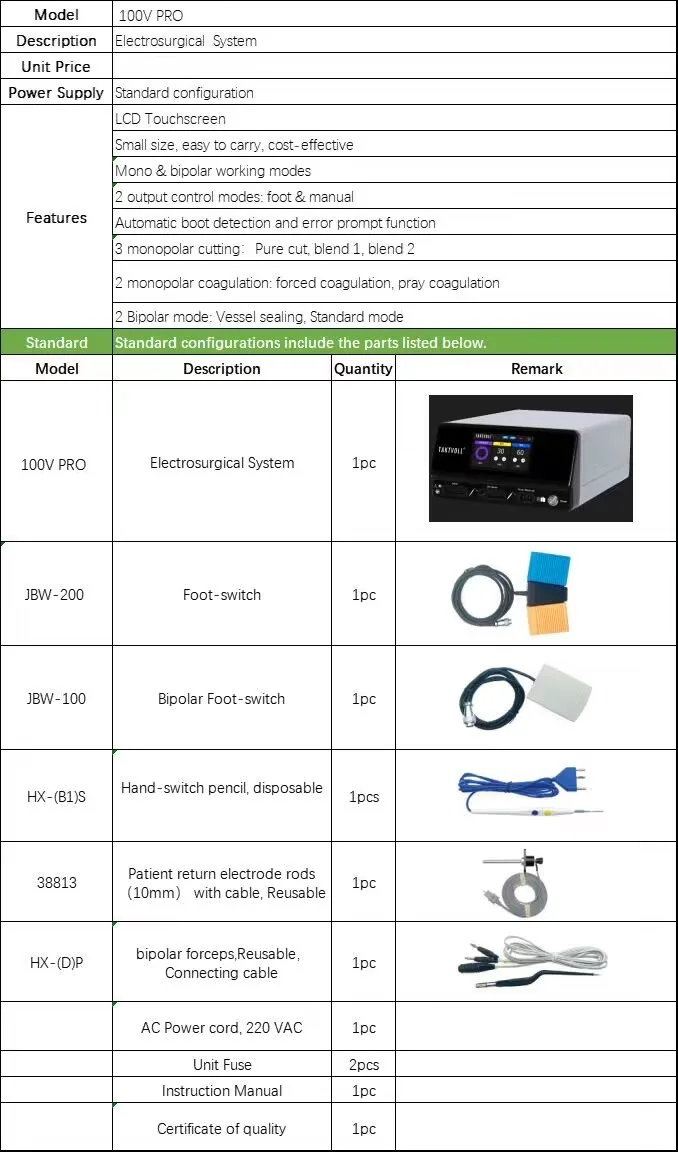
Power: 100W; Weight: 4.7 kg; Dimensions: 330(L) x 280(W) x 100(H) mm; Instrument classification: Class III; Power source: electricity; Place of origin: Anhui, China; Quality certification: CE. Shelf life is listed as 1 year. For mains voltage and frequency please consult the user manual or supplier.
4. What working modes does the MT 100V Pro offer?
It offers up to four working modes: Pure Cut, Blend Cut, Standard Coagulation, and Spray Coagulation, allowing selection based on tissue effect and clinical need.
5. Is the unit monopolar or bipolar?
The MT 100V Pro is described as a monopolar cautery machine. Use requires a monopolar active electrode (handpiece/electrode tip) and a return (neutral) electrode pad placed on the patient.
6. What safety features are included?
Built-in safety features include a return electrode monitoring system to reduce risk of burns at the return site, a 5-second automatic self-inspection on power-up, visible mode indications, adjustable operation sounds to warn of misoperation, and minimized tissue sticking/carbonization to reduce surgical damage.
7. How do I start and operate the device safely?
Basic steps: place and secure the patient return electrode, connect the handpiece and required electrodes, power on (unit runs a 5-second auto inspection), select the appropriate mode and power setting, and perform cautery only by trained clinicians familiar with electrosurgery. Always follow the supplied user manual and institutional electrosurgery safety protocols.
8. What consumables and accessories are required?
Typical required items include sterile monopolar electrode tips, a compatible monopolar handpiece, and a patient return (neutral) electrode pad. Specific part numbers and compatible consumables should be obtained from the manufacturer or authorized supplier.
9. How should the unit be cleaned and maintained?
Disconnect and power off before cleaning. Wipe external surfaces with a manufacturer-approved disinfectant; do not immerse the unit or allow liquid into vents/connectors. Inspect cables, connectors and pads regularly. Follow the manufacturer's maintenance schedule and local biomedical engineering procedures for electrical safety and performance checks.
10. Is the MT 100V Pro certified for clinical use?
The product listing shows CE certification, indicating conformity with relevant European safety and performance requirements. It is classified as a Class III instrument. Verify local regulatory requirements and approvals for use in your region.
11. What is the shelf life and warranty information?
The product listing states a shelf life of 1 year. Warranty details are not provided in the description—please contact the manufacturer or your supplier for specific warranty terms, service agreements, and RMA procedures.
12. Are OEM or customization options available?
Yes. The listing indicates OEM projects are welcome; contact the manufacturer or sales representative to discuss custom branding, configuration, or localized feature requirements.
13. What precautions should be taken to prevent patient burns or interference?
Ensure correct placement and good contact of the return electrode, avoid placing return pads over metallic implants or bony prominences, remove flammable agents or oxygen-rich environments near the surgical site, and follow electromagnetic compatibility guidance to avoid interference with other devices.
14. How often should the unit be serviced or calibrated?
Periodic preventive maintenance and electrical safety testing are recommended per institutional policies and the manufacturer's instructions. Frequency depends on usage intensity; consult the service manual or supplier for recommended intervals.
15. Who should use this device?
The MT 100V Pro is intended for use by qualified medical professionals (surgeons, operating room staff) trained in electrosurgical techniques and device-specific operation. It is not for lay use.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading