B21, China Town Mall, Midrand
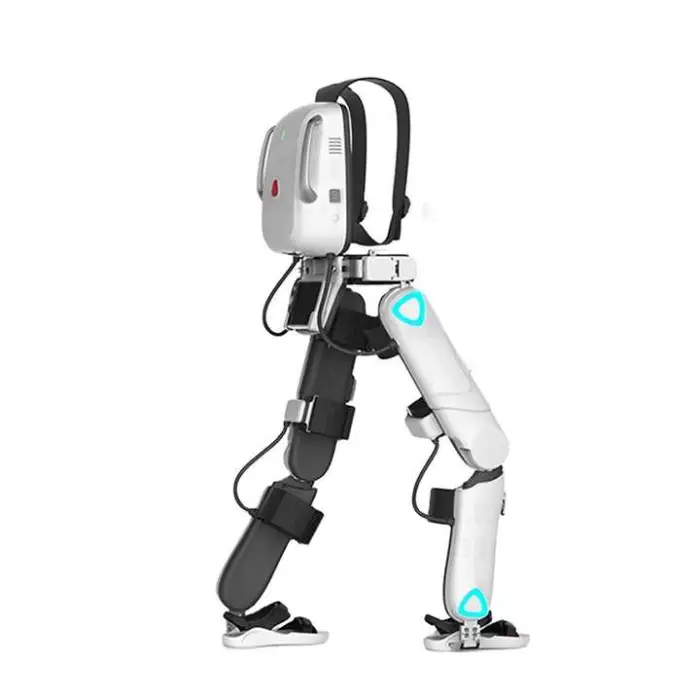
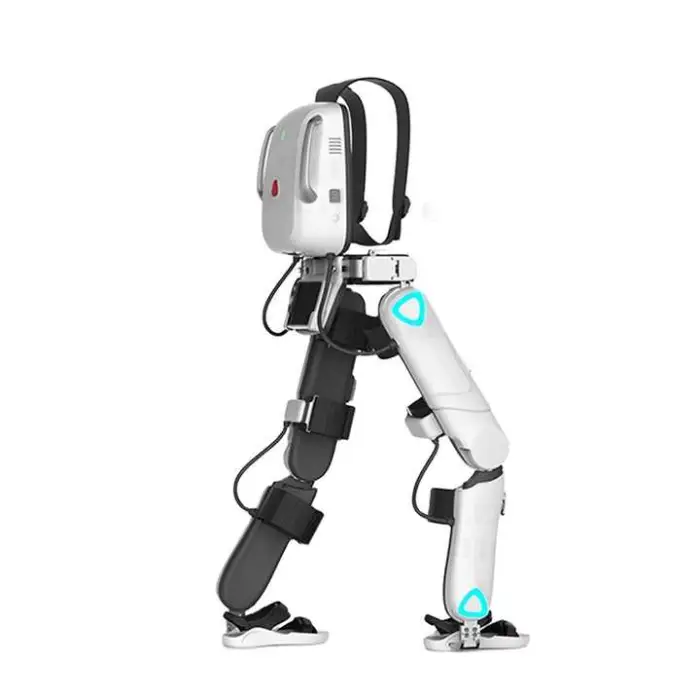
Medical Lower Limb Rehabilitation Exoskeleton Robot Suit Physical Therapy Gait Training Equipment
- Section : Medical Supplies
- Category : Rehabilitation Equipment
- SKU : 1600149112987

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 05 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Medical Lower Limb Rehabilitation Exoskeleton Robot Suit?
This is an assistive exoskeleton designed for lower limb rehabilitation and gait training. It helps users practice walking patterns under guided support and is intended for use in physical therapy clinics, rehabilitation centers, research, and supervised home therapy.
2. Who is the device intended for?
The device is intended for individuals who require gait training or lower limb rehabilitation, including patients recovering from surgery or injury, elderly people seeking mobility improvement, and participants in rehabilitation research. Use should be determined by a qualified clinician.
3. What are the applicable height and weight limits?
The exoskeleton is adjustable for users with heights between 150 cm and 190 cm and supports a maximum user weight of up to 85 kg.
4. What training modes are available and what is the walking speed?
The device offers three modes: Weight Loss Mode, Training Mode, and Smart Mode. The nominal walking speed is 2 km/hr. For mode selection and parameter tuning, follow clinical guidance or the user manual.
5. Is the device safe to use and are there built-in safety features?
The suit is designed for therapeutic use and includes safety considerations typical of rehabilitation exoskeletons. However, safe operation requires trained supervision, correct fitting, and adherence to the user manual. Always use under the guidance of a clinician or qualified therapist.
6. Are there any contraindications or patients who should not use the exoskeleton?
Specific contraindications depend on the user’s medical condition. Common reasons to avoid exoskeleton use include unstable fractures, uncontrolled cardiopulmonary or acute medical conditions, severe joint instability, or any condition deemed unsuitable by a clinician. Consult a healthcare professional before use.
7. Can this exoskeleton be used at home?
The product may be used in home therapy settings for convenient rehabilitation, but home use should be approved and supervised by a qualified clinician or trained caregiver to ensure safety and appropriate training protocols.
8. Do I need special training to operate the device?
Yes. Operators and therapists should receive training on device setup, fitting, mode selection, safety checks, and emergency procedures. The manufacturer or authorized distributor can provide training resources or onsite instruction.
9. What are the power requirements and battery specifications?
Power and battery specifications are not listed here. Please consult the product manual or contact the manufacturer/distributor for detailed information about power supply, battery type, runtime, and charging procedures.
10. How should the exoskeleton be cleaned and maintained?
Perform routine visual inspections, tighten fasteners as needed, and clean external surfaces regularly with a mild disinfectant recommended by the manufacturer. Do not immerse electrical components in liquid. Follow the maintenance schedule and procedures in the user manual and contact service support for repairs.
11. What warranty and after-sales service are provided?
The product offers a service guarantee of three years for return and replacement. For warranty claims, repairs, or replacement parts, contact the seller or the ICLEAR service team and provide purchase and product information as requested.
12. What is the instrument classification of the device?
The device is listed as Instrument Classification: Class I, indicating a low-risk medical device category in many regulatory frameworks. Check local regulations for specific classification and approval requirements in your region.
13. What are the package dimensions and shipping weight?
Single package size is 120 x 70 x 70 cm and the single gross weight is 50.000 kg.
14. Are there accessories or compatible devices available?
Standard accessory details are not provided here. For information on available accessories, spare parts, compatible sensors, or integration with gait analysis systems, contact the manufacturer or authorized distributor.
15. How can I arrange a demo, purchase, or get technical support?
To arrange a demonstration, request a quote, order the device, or obtain technical support, contact the manufacturer ICLEAR or an authorized distributor. They can provide local sales contacts, training options, service agreements, and product documentation.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading