B21, China Town Mall, Midrand
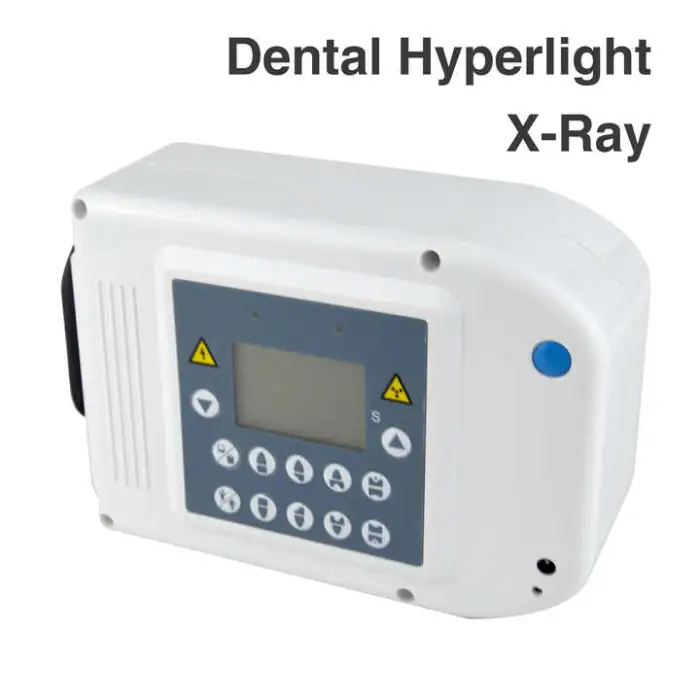
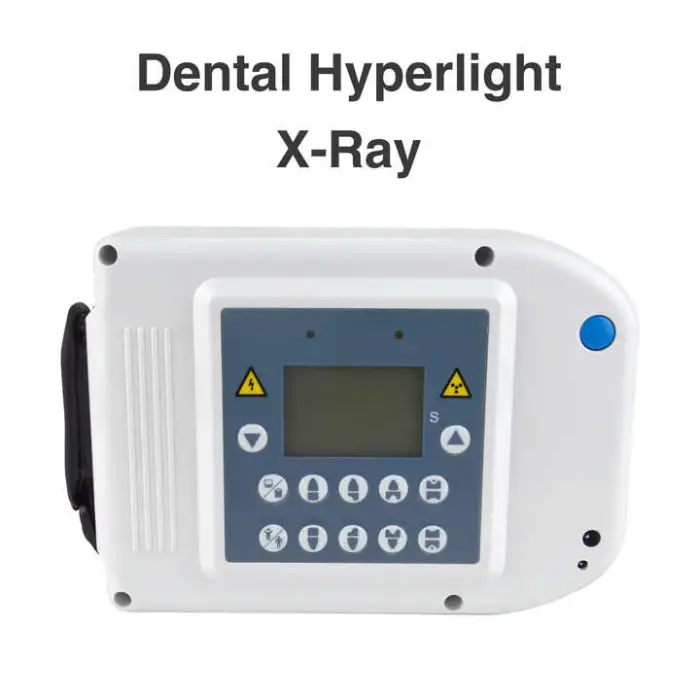
Dental Hyperlight X-Ray Oral Therapy Equipment & Accessory
- Section : Medical Supplies
- Category : Dental Handpiece
- SKU : 1601371733375

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 05 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the Dental Hyperlight X-Ray Oral Therapy Equipment & Accessory?
The Dental Hyperlight X-Ray (Model M-L88, brand HDR) is an electric dental X‑ray imaging device designed to produce clear images of teeth and gums for diagnosis and treatment planning. It is a Class I medical instrument with CE quality certification and a stated shelf life of 3 years.
2. What does the 'Class I' instrument classification mean?
Class I indicates a low‑to‑moderate risk medical device under common regulatory frameworks. It generally requires compliance with applicable safety and labeling rules but typically involves fewer regulatory controls than higher‑risk devices.
3. Is this product certified for quality and safety?
Yes. The product description lists CE quality certification, which indicates conformity with relevant European safety, health and environmental protection requirements. You should also follow local regulations for X‑ray equipment use.
4. What power source and electrical requirements does it have?
The device is electric. Specific voltage, current and plug type requirements are not listed in the summary — request the technical datasheet or contact the supplier to confirm mains specifications and site preparation needs before purchase.
5. What is included with the purchase — does it come with accessories?
The product page lists selling units as a single item and describes accessories in the product name. Exact accessory contents (e.g., positioning devices, cables, mounts) are not specified here — contact the seller or check the full product packing list for details.
6. How do I install the Dental Hyperlight X‑Ray unit?
Installation typically requires following the manufacturer's instructions and ensuring proper electrical connections. Professional installation and site readiness checks are recommended to meet safety and radiation shielding requirements. Ask the supplier for an installation manual or on‑site support options.
7. Is technical support available?
Yes. The product description states online technical support is available. For details about hours, response times and service scope, confirm with the vendor or authorized distributor.
8. What maintenance and cleaning are required?
Routine maintenance usually includes visual inspections, cleaning external surfaces with appropriate non‑abrasive disinfectants, periodic functional checks and calibration as recommended by the manufacturer. Do not immerse the unit in liquids. Obtain the maintenance schedule and cleaning instructions from the user manual or supplier.
9. What does the 3‑year shelf life mean?
A 3‑year shelf life indicates the period the manufacturer guarantees the device or certain components retain expected performance when stored under recommended conditions. Before putting the unit into clinical service after storage, follow the manufacturer's pre‑use checks and, if necessary, request functional testing.
10. Is the device safe in terms of radiation exposure?
The unit carries CE certification which implies it meets applicable safety standards, but safe use depends on correct operation, shielding and adherence to local radiation protection regulations. Operators should follow dose‑minimization practices, use protective gear and ensure proper training and monitoring.
11. Who should operate the Dental Hyperlight X‑Ray equipment?
It should be operated by trained dental professionals or radiography‑qualified staff. Although the product is described as easy to operate, personnel should receive training in device operation, image acquisition protocols and radiation safety.
12. Can this X‑ray unit be used for pediatric patients?
Yes — it can be used for dental imaging of both adults and children. When imaging pediatric patients, follow pediatric exposure settings, use appropriate shielding and adhere to the ALARA (as low as reasonably achievable) principle to minimize dose.
13. What image quality and file compatibility can I expect?
The product is described as producing high‑quality images for accurate diagnosis. Specific image resolution, output formats and digital interface compatibility are not listed here — request the technical specifications or demo from the supplier to confirm compatibility with your practice management or imaging software.
14. What warranty, repairs and spare parts support are available?
Warranty and repair policies are not specified in the provided description. Contact the manufacturer or authorized distributor for details on warranty length, coverage, repair services, spare parts availability and service contracts.
15. How can I get a quote, demo, or place an order?
Contact an authorized HDR distributor or the seller listed on the product page to request a quote, technical datasheet, live demo or to place an order. Ask about shipping, lead times, installation services and any required regulatory paperwork for your region.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading