B21, China Town Mall, Midrand
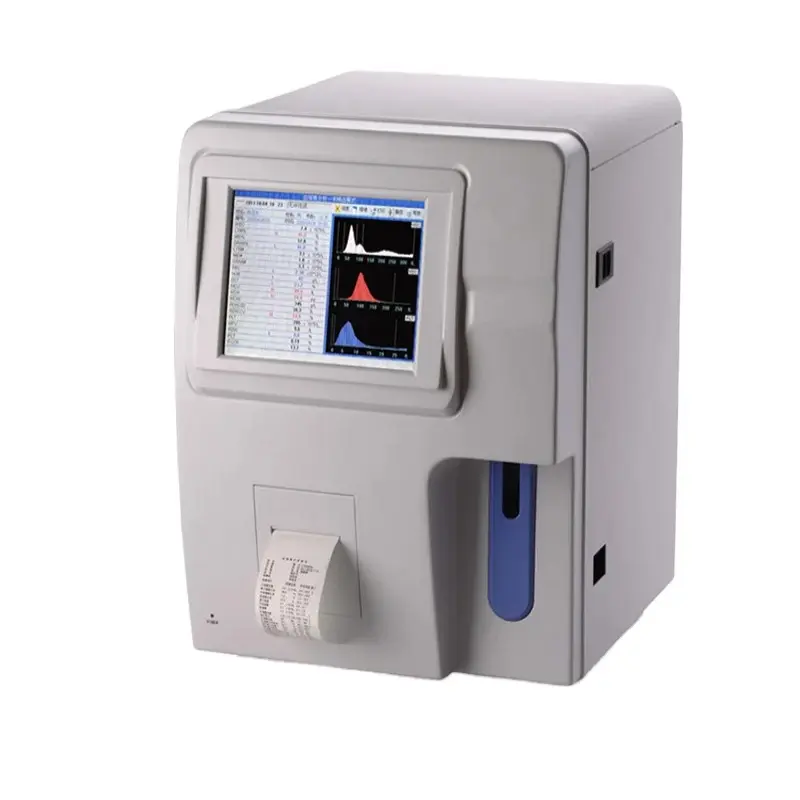
Blood Testing Equipment – RSD3000 Model
- Section : Medical Supplies
- Category : Clinical Analytical Instruments
- SKU : 62305862699

- Shipping Timeframes: All orders are processed within 2-5 business days (excluding weekends and holidays). After your order has been processed, the estimated delivery time is before 05 May, 2026, depending on customs, Please note that due to high demand, some items may experience longer shipping times, which will be communicated at order confirmation email.
- Order Processing Time: Please allow 2-5 business days for us to process your order before it is shipped . Orders placed after 16:00 on Fridays, or during weekends and public holidays, will begin processing on the next business day. Processing times may be extended during peak seasons or sales events.
- Manufacturing Time: Some products needs manufacturing time, the manufacturing process will take approximately 10-30 business days depending on the product. This timeframe may vary depending on the complexity of the product and current demand. but this will be communicated with you during order confirmation.
- Returns and Exchanges: We offer a 30-day return policy for most items. If you are not completely satisfied with your purchase, you may return it within 30 days of receipt for a refund or exchange. Items must be unused, in their original packaging, and accompanied by proof of purchase. Return shipping costs are the responsibility of the customer, unless the item was damaged or defective upon arrival.
1. What is the RSD3000 Blood Testing Equipment and who is it intended for?
The RSD3000 is an advanced, Class II blood testing device from RONSEDA designed for quick and accurate blood analysis. It is intended for use in medical laboratories, hospitals and clinics that require reliable, high-volume blood testing solutions.
2. Which safety standards and certifications does the RSD3000 meet?
The RSD3000 complies with the EN 149 -2001+A1-2009 safety standard and is CE certified, indicating conformity with relevant European health, safety and environmental protection requirements.
3. How many parameters can the RSD3000 analyze?
The device is capable of analyzing 23 different blood parameters, providing a comprehensive panel for routine clinical testing.
4. What are the device's display and user interface features?
The RSD3000 features an 8.4-inch large LCD digital display and a user-friendly interface designed to make operation and result interpretation straightforward for healthcare professionals.
5. What types and volumes of samples does the RSD3000 require?
The RSD3000 supports venous and prediluted samples. Minimum sample volumes are 9.8 µL for venous samples and 20 µL for prediluted samples (sufficient to perform two tests in one session).
6. How much data can the RSD3000 store?
The device can store up to 10,000 sample records and up to 3 histograms, making it suitable for high-volume testing environments that require local retention of patient data.
7. How do I connect the RSD3000 to a computer or laboratory information system (LIS)?
The RSD3000 includes an RSD232 serial interface for connection to a PC. For integration with an LIS or specific software, contact RONSEDA or your authorized dealer to confirm compatibility and required settings.
8. Is the RSD3000 electrically powered and suitable for continuous use?
Yes, the RSD3000 is electrically powered and intended to provide consistent performance for high-volume testing environments. Refer to the product manual for specific power ratings and installation requirements.
9. What warranty and after-sales support are provided with the RSD3000?
The RSD3000 comes with a 1-year warranty. For repairs, calibration, technical support or warranty claims, contact RONSEDA or your authorized distributor with the device serial number and proof of purchase.
10. What is the shelf life of the RSD3000?
The product specification lists a shelf life of 2 years. For long-term storage, transport, and deployment recommendations, follow RONSEDA's storage guidelines in the product documentation.
11. How should the RSD3000 be maintained and calibrated?
Routine maintenance and calibration should follow the manufacturer's instructions and local regulatory requirements. Regular checks, periodic calibration, and use of approved reagents and consumables are recommended to ensure accuracy and reliability.
12. Can I export or back up stored results and histograms?
Yes. Stored data can be transferred to a PC through the RSD232 interface for record keeping and backup. For details on supported export formats and backup procedures, consult the user manual or contact RONSEDA support.
13. What consumables and reagents are required, and where can I order them?
The RSD3000 requires specific reagents and consumables compatible with the instrument. Use RONSEDA-approved reagents and replace consumables per the product manual. Contact RONSEDA or an authorized distributor to order supplies and get part numbers.
14. Are there any training or user requirements before operating the RSD3000?
While the RSD3000 has a user-friendly interface, initial user training is recommended to ensure correct operation, sample handling, quality control procedures and data management. Training can be arranged through RONSEDA or an authorized service partner.
Latest Order Arrivals
Discover our latest orders

12 Heads Embroidery Machine

Dewatering Pump Machine

Order Collection

Portable Water Drilling Rig

Order Usefully Collected

Batch of Orders

Agriculture Processing Machines

Meat Grinder Machine

Water Pump Equipment

Packaging Machine and accessories

Fabrics Manufacturing Equipment

Mining Equipments

Food Processing Machine

Batch of Orders

Batch of Orders

Latest Orders Labelled

wheel alignment machines

new arrivals

Pre Orders Offloading

Latest Arrivals

Latest Arrivals

Latest Arrivals

26 January 2026

Toilet paper making machine

Toilet paper making machine

Toilet paper Rewinding Machine

latest arrivals

offloading

order success

order collection

order offloading